Standard EMF of a Cell
Apr 28, 2022, 16:45 IST
What is SHE
The potential of any electrode is the potential difference between it and the electrolyte surrounding the electrode. The electrode potential depends upon the nature of the metal, concentration of the metallic ions in solution and the temperature of the solution. When the ions are at unit activity and the temperature is 250C (298K), the potential difference is called a standard electrode potential (E0). The potential of a single electrode can not be determined but the potential difference between the two electrodes can be accurately measured. Standard hydrogen electrode (SHE), is used as the standard reference electrode. By convention, the electrode potential of SHE has been assigned a value of zero volt.
Thus the standard electrode potential of a metal may be defined as the potential in volts developed in a cell consisting of two electrodes , the pure metal in contact with a molar solution of its ions andSHE.
Positive and negative electrode potentials: If on combining an electrode A with the SHE, a reduction process (gain of electrons) occurs at the electrode, the potential of the electrode is called as reduction potential . It is given a positive sign as reduction electrode potential or a negative sign as oxidation electrode potential. On the other hand, if on combining an electrode B with the SHE, an oxidation process (loss of electrons) occurs at B, the electrode potential (half - cell potential) is called as the oxidation potential of electrode B. It is given a positive signs as oxidation electrode potential and a negative sign as reduction electrode potential. Thus in short,
Standard oxidation potential for any half cell = - (Standard reduction potential)
Standard reduction potential for any half cell = - (Standard oxidation potential)
USES OF ELECTRODE POTENTIAL
- It predicts relative reducing strength of reducing agents. We know that a reducing agent is a substance which has tendency to donate electrons in chemical reactions . Hence Reducing strength preopertional to Tendency to donate electron and Tendency to get oxidized. Thus higher the standard reduction potential of a species, lesser will be its reducing strength. Thus Li having least reduction potential is the strongest reducing agent in aqueous solution, and having maximum reduction potential is the weakest reducing agent.
-
It predicts the relative activity of metals. Those elements which have a tendency to form cations are known as metals. Hence Reactivity of a metal Tendency to form cation Tendency to lose outer electrons
. Thus lesser the standard reduction potential of an element (metal), greater will be its activity. Thus elements of group 1 having very high values of standard oxidation potential (low value of reduction potentials) are highly reactive and hence found only in combined state.
-
It predicts whether a metal can displace hydrogen gas from a hydracid or not. Hydracids posses hydrogen as H+ ions which are in need of electrons to get converted into hydrogen atoms which then combine to form H2 . Hence for hydracids to evolves H2 gas , these must be treated with electron donor element (metal). Thus only those metals which have positive value of standard oxidation potential ( or negative value of
) will be able to donate e- to H+ of hydracids forming H2 gas.
- An oxidizing agent witha higher reduction potential will oxidize any reducing agent with a lower eduction potential.
- A reducing agent with a lower reduction potential will reduce any oxidising agent with a higher reduction potential.
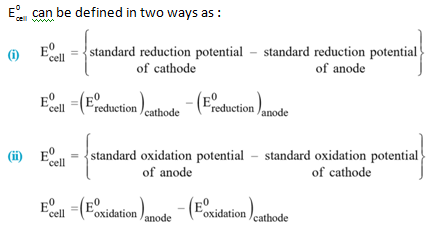
Standard EMF of a Cell (E 0 cell ) :