
Formula For Vant Hoff Factor
Sep 06, 2022, 16:45 IST
Van’t Hoff Factor:
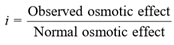
Degree of Association :
It is the fraction of total number of molecules of solute which combines to form bigger molecules. Let n moles of solute (X) associate from one mole of it.
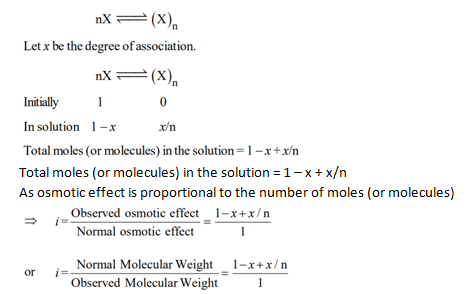
Degree of Dissociation :
It is defined as the fraction of total number of molecules which dissociates into free ions.
Let us take 1 mole of KCl and x be its degree of dissociation, then we have
Example For Van't Hoff Factor
Q1. Value of van’t Hoff factor for K 2 SO 4 solution with 50% dissociation?
Ans.
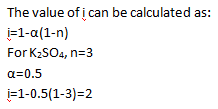
Q2. Assuming complete dissociation, which one of the following will have a van't Hoff factor of 2?
A. MgSO 4
B. Sucrose
C. H 2 SO 4
D. Lead nitrate
Ans. Correct option is A
Q3. From a measurement of the freezing-point depression of benzene, the molecular weight of acetic acid in a benzene solution was determined to be 100. The percentage association of acetic acid is
A. 79%
B. 93%
C. 80%
D. 100%
Ans. Correct option is C
For More C hemistry Formulas just check out main page of Physics Wallah









