
NCERT Solutions for Class 10 Science Chapter 2: The NCERT Solutions for Class 10 Science Chapter 2 offer detailed explanations for each question in the textbook, ensuring clarity in fundamental concepts. They present answers in a step-by-step format, which helps students tackle theoretical as well as numerical problems efficiently.
The NCERT Solutions for Class 10 Science Chapter 2 are designed to help students master the material, align with the CBSE exam pattern, and provide a strong foundation for future studies in chemistry. They are an essential resource for exam preparation and concept reinforcement.NCERT Solutions for Class 10 Science Chapter 2 Overview
NCERT Solutions for Class 10 Science Chapter 2, "Acids, Bases, and Salts," provide a comprehensive guide to understanding the properties and behavior of these substances. The chapter explains key concepts such as the nature of acids and bases, the role of indicators in distinguishing them, and the pH scale, which measures the acidity or basicity of a solution. It covers important reactions, including neutralization between acids and bases, and delves into the preparation and properties of salts. NCERT Solutions for Class 10 Science Chapter 2 also highlights the importance of pH in everyday life, explaining its significance in various contexts like agriculture, human body functions, and industrial applications. Additionally, students learn about the preparation and use of common salts such as sodium chloride, baking soda, and washing soda.NCERT Solutions for Class 10 Science Chapter 2 PDF
The NCERT Solutions for Class 10 Science Chapter 2 pdf provide step-by-step answers to both theoretical and numerical problems, following the NCERT syllabus. NCERT Solutions for Class 10 Science Chapter 2 help students build a solid understanding of core chemistry topics, supporting effective exam preparation and strengthening problem-solving skills.NCERT Solutions for Class 10 Science Chapter 2 PDF
NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases, and Salts
Below we have provided NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases, and Salts -1. You are given three test tubes. The three test tubes contain distilled water, an acidic solution and the basic solution, respectively. There is only red litmus paper available in order to identify what is there in each test tube. How will you find out what is in each of the test tubes?
Solution:
Using red litmus paper, we can determine what is in each test tube. This can be accomplished by observing how the red litmus paper changes colour.- The three solutions in the test tubes are poured one at a time onto litmus paper.
- A basic answer can be found in the solution that changes red litmus to blue.
- The blue litmus paper should be divided into two sections.
- The acidic solution is the one in the test tube that causes the blue litmus paper to become red.
- Water is included in the test tube solution, which does not alter the colour of the red or blue litmus paper.
In-text questions set 2 — Page number 22
1. Why should curd and sour substances not be kept in brass and copper vessels?
Solution:
Acidic materials, such as curd and sour culinary ingredients, react with metal. Food becomes poison as a result of this process, endangering people's health.2. Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Solution: When an acid reacts with any metal, salt and hydrogen gas are formed.
Metal + Acid → Salt + Hydrogen gas3. Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Solution:
Here, CO2 is the gas that has evolved since calcium chloride, a metal compound, was liberated. Therefore, calcium carbonate should be metal A. As a result, the process involving calcium carbonate and HCl is CaCO 3 (s) + 2HCl (Aq) → CaCl 2 ( Aq) + CO 2 (g) + H 2 O (l)In-text questions set 3 Page number – 25
1. Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show an acidic character?
Solution:
When H+ ions are released into water, a substance becomes acidic or non-acidic. Acids are chemicals that produce hydrogen ions when they dissociate with water. When certain substances breakdown in an aqueous solution and produce hydrogen ions, or acids like HCl and HNO3, they exhibit an acidic nature. While they do contain a hydrogen atom, compounds like glucose and alcohol do not exhibit any evidence of being acidic. The truth is that, unlike the hydrogen in acids, the hydrogen in them will not separate. They will dissolve in the water without splitting into hydrogen ions.2. Why does an aqueous solution of acid conduct electricity?
Solution:
An acid's ability to conduct electricity is due to charged particles. Acid has electrical conductivity due to these charged particles, also known as ions.3. Why does dry HCl gas not change the colour of the dry litmus paper?
Solution: HCl does not give out Hydrogen ions; therefore, HCl does not show any acidic behaviour, and the colour of the litmus paper remains the same on reacting with HCl gas.
4. While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Solution:
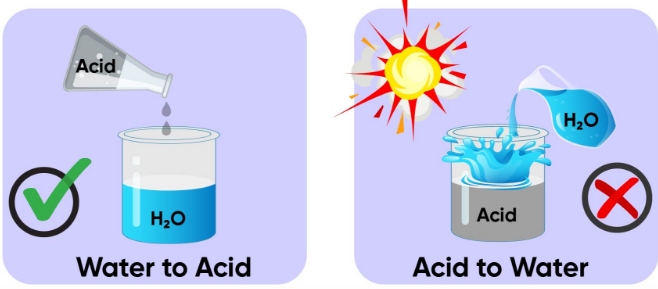 It is advised to dilute acids by adding the acid to water rather than the water to the acid. This is because adding water to a concentrated acid generates a lot of heat, which can explode and burn the skin, clothing, and other body parts. Therefore, adding acid to water is safe, while adding water to acid is not.
It is advised to dilute acids by adding the acid to water rather than the water to the acid. This is because adding water to a concentrated acid generates a lot of heat, which can explode and burn the skin, clothing, and other body parts. Therefore, adding acid to water is safe, while adding water to acid is not.
5. How is the concentration of hydronium ions (H 3 O + ) affected when a solution of an acid is diluted?
Solution:
There will be a fixed quantity of hydronium in the fixed volume of the solution when acid is introduced to water. Hydronium concentration in the solution drops as a result of a decrease in the number of hydronium ions per volume of the solution when it is diluted.6. How is the concentration of hydroxide ions (OH – ) affected when excess base is dissolved in a solution of sodium hydroxide?
Solution:
A base's hydroxide ions rise when it dissolves in sodium hydroxide solution, but eventually it reaches saturation. Even when additional base is added, the hydroxide ion concentration remains unchanged beyond the saturation point.In-text questions set 4 Page number – 33
1. You have two solutions, A and B. The pH of solution A is 6, and the pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of these is acidic, and which one is basic?
Solution:
The principle that "The pH of any solution is inversely proportional to the hydrogen ion concentration" can be used to determine the hydrogen ion concentration. It follows that a solution with a lower pH will have a higher concentration of hydrogen ions. As a result, solution A will contain more hydrogen ions. Furthermore, solution A will be acidic and solution B will be basic.2. What effect does the concentration of H + (aq) ions have on the nature of the solution?
Solution:
The concentration of hydrogen ions determines the kind of solution. The solution becomes acidic as the concentration of hydrogen ions rises, and it becomes basic when the concentration of hydrogen ions falls.3. Do basic solutions also have H +( aq) ions? If yes, then why are these basic?
Solution: Basic solutions have H + ions, but hydroxide ions present in basic solution are more in basic solution. Hence, Hydroxide ions turn the solution into basic.
4. Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide), or chalk (calcium carbonate)?
Solution:
Fields with acidic soil (pH less than 7) should be treated with chalk (calcium carbonate), slaked lime (calcium hydroxide), or quick lime (calcium oxide).In-text questions set 5 Page number – 34-35
1. What is the common name of the compound CaOCl 2 ?
Solution: The common name of CaOCl 2 is bleaching powder.
2. Name the substance, which on treatment with chlorine, yields bleaching powder.
Solution: The substance, which on treatment with chlorine, yields bleaching powder is Calcium hydroxide.
3. Name the sodium compound which is used for softening hard water.
Solution: Sodium carbonate is the compound which is used for softening hard water.
4. What will happen if a solution of sodium hydrocarbonate is heated? Give the equation of the reaction involved.
Solution: Heating sodium hydrocarbonate yields sodium carbonate, and carbon dioxide gas is liberated in the process.
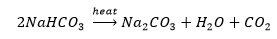
5. Write an equation to show the reaction between Plaster of Paris and water.
Solution: The chemical equation for the reaction of Plaster of Paris and water is
CaSO 4 .1/2H 2 O + 3/2H 2 O → CaSO 4 .2H 2 OExercise questions Page number – 33
1. A solution turns red litmus blue, its pH is likely to be
a) 1 (b) 4 (c) 5 (d) 10
Solution: The answer is 10 because litmus paper turns blue when the solution reacts with a basic solution (PH more than 7). Hence, 10 is the answer.
2. A solution reacts with crushed eggshells to give a gas that turns lime-water milky. The solution contains
a) NaCl (b) HCl (c) LiCl (d) KCl
Solution: The answer is HCl.
Eggshells contain calcium carbonate, which on reaction with HCl, liberates CO 2 gas, which turns lime water into milky. CaCO 3 + 2HCl → CaCl 2 + H 2 O + CO 23. 10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralise it will be
(a) 4 mL (b) 8 mL (c) 12 mL (d) 16 mL
Solution: Since 10 ml of NaOH requires 8 mL of HCL, 20 ml of NaOH requires 8 x 2 = 16mL of HCl. Hence, the answer is option (d) 16mL.
4. Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic (b) Analgesic (c) Antacid (d) Antiseptic
Solution: Indigestion is due to the excess production of acid in the stomach. Medicines used to treat indigestion is called Antacid.
5. Write word equations and then balanced equations for the reaction taking place when
(a) Dilute sulphuric acid reacts with zinc granules.
(b) Dilute hydrochloric acid reacts with magnesium ribbon.
(c) Dilute sulphuric acid reacts with aluminium powder.
(d) Dilute hydrochloric acid reacts with iron filings.
Solution:
(a) Dilute sulphuric acid reacts with zinc granules.=> Dilute sulphuric acid + zinc → Zinc Sulphate + Hydrogen Gas
=> H 2 SO 4 (aq) + Zn → ZnSO 4 (aq) + H 2 (g)
(b) Dilute hydrochloric acid reacts with magnesium ribbon.=> Dilute Hydrochloric + Magnesium → Magnesium Chloride + Hydrogen Gas
=> 2HCl(aq) + Mg → MgCl 2 (aq) + H 2 (g)
(c) Dilute sulphuric acid reacts with aluminium powder.=> Dilute Sulphuric Acid + Aluminium → Aluminium Sulphate + Hydrogen Gas
=> 3H 2 SO 4 (aq) + 2Al(s) → Al 2 (SO 4 ) 3 (aq) + 3H 2 (g)
(d) Dilute hydrochloric acid reacts with iron filings.=> Dilute Hydrochloric Acid + Iron → Ferrous Chloride + Hydrogen Gas
=> 6HCl(aq) + 3Fe(s) → 3FeCl 2 (aq) + 3H 2 (g)
6. Compounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
Solution:
As illustrated in the figure, drive two nails into the wooden or rubber cork and set them on a beaker. Attach the iron nail to a wire that is attached to the switch, a 6-volt battery, and a lightbulb. To dip the nails in alcohol or glucose, pour some of each substance. When you turn on the switch, even if the lightbulb is connected to the switch, it does not shine. After emptying the beaker, pour the HCL solution in. The lightbulb lights this time. This demonstrates that whereas alcohol and glucose do not carry electricity, acid does.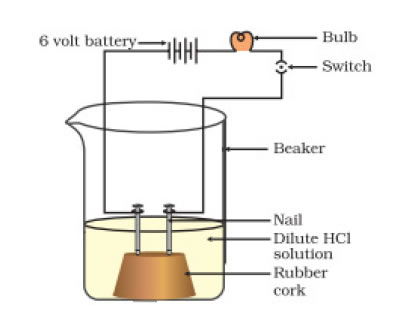
7. Why does distilled water not conduct electricity, whereas rain water does?
Solution:
- There are no ionic compounds found in distilled water.
- Rainwater, on the other hand, contains far more chemicals.
- Carbonic acid is created when acidic gas, such as carbon dioxide from the atmosphere, dissolves in rainwater. This indicates that it contains carbonate and hydrogen ions. Rainwater can therefore conduct electricity when it contains acids.
8. Why do acids not show acidic behaviour in the absence of water?
Solution:
The existence of hydrogen ions is the cause of acids' acidic nature. Since water is necessary for the production of hydrogen ions, it is imperative that water be present for acids to exhibit their acidic properties.9. Five solutions, A, B, C, D and E, when tested with a universal indicator, showed pH as 4, 1, 11, 7 and 9, respectively. Which solution is
(a) Neutral?
(b) Strongly alkaline?
(c) Strongly acidic?
(d) Weakly acidic?
(e) Weakly alkaline?
Solution: In increasing order of hydrogen ion concentration,
pH 11(C) < pH 9(E) < pH 7 (D) < pH 4 (A) < pH 1 (B) PH11 – Strongly alkaline pH9 – Weakly alkaline PH7 – Neutral pH4 – Weakly acidic pH1 – Strongly acidic10. Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH 3 COOH) is added to test tube B. Amount and concentration taken for both the acids are the same. In which test tube will the fizzing occur more vigorously and why?
Solution:
While acetic acid is a weaker acid, HCl is a stronger acid. The hydrogen gas produced as a result of the acid's reaction with the magnesium ribbon is what causes the fizzing. Test tube A is releasing a lot of hydrogen gas since HCl is a very strong acid. Test tube A consequently experiences increased fizzing.11. Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
Solution: Fresh milk is turned to curd due to the production of lactic acid. Lactic acid reduces the pH of the milk.
12. A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
(b) Why does this milk take a long time to set as curd?
Solution: (a) He shifted the pH of the fresh milk from 6 to slightly alkaline to prevent milk from getting sour due to the production of lactic acid.
(b) This milk takes a long time to set into curd because the lactic acid produced here first neutralises the pH, and then the pH is reduced to turn the milk into curd.
13. Plaster of Paris should be stored in a moisture-proof container. Explain why.
Solution:
Because moisture might cause hydration, which slows down the setting of the plaster, plaster of paris should be stored in a moisture-proof container. Plaster will become useless as a result.14. What is a neutralisation reaction? Give two examples.
Solution: The reaction of the acid + base gives a product of salt + water, which is considered a neutralisation reaction.
Examples: NaOH + HCl → NaCl + H 2 O Mg(OH) 2 + H 2 CO 3 → MgCO 3 + 2H 2 O15. Give two important uses of washing soda and baking soda.
Solution:
| Washing soda | Baking soda |
| 1. It is used as an electrolyte | 1. It can be used to test the garden soil for acidity. If bubbles are developed, then the soil is too acidic |
| 2. It can be used domestically as a water softener for laundry. | 2. If used on washing the car, then it will remove dead bug bodies without damaging the colour or the paint on the car. |













