
The aluminium bromide formula is a chemical compound formed by combining aluminium and bromine. Its chemical formula and ionic nature are essential for understanding its properties and applications.
Aluminium bromide melts into a colourless liquid as a white crystalline compound when heated. Furthermore, aluminium bromide is hygroscopic. It mostly exists as a dimeric compound in the solid phase. Friedel-Crafts alkylation is performed using this compound as a catalyst.
Moreover, it belongs to a class of inorganic compounds referred to by experts as post-transition metal bromides. Further, aluminium bromide has a whitish to yellow-red color, with the largest halogen atom being Bromine. A pure state of aluminium bromide is more whitish in color.
Aluminium Bromide Formula
The aluminium bromide formula is AlBr3. This formula represents the composition of aluminium bromide as consisting of one aluminum cation (Al^3+) and three bromide anions (Br^-). This compound is classified as an ionic compound, which means it is held together by strong electrostatic forces between the positively charged aluminium ions and the negatively charged bromide ions.
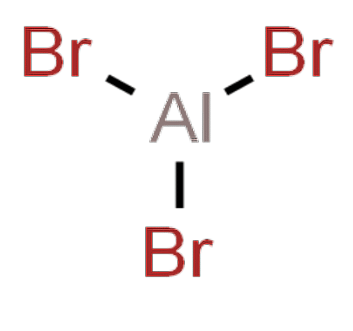
Aluminium Bromide Formula - Ionic Compound
The aluminium bromide formula is a classic example of an ionic compound. Ionic compounds are formed when atoms transfer electrons to achieve a stable, full outer electron shell. In the case of aluminium bromide, aluminium loses three electrons to become a positively charged ion (Al^3+), while bromine gains one electron to become a negatively charged ion (Br^-). The strong attraction between these oppositely charged ions leads to the formation of the ionic compound AlBr3. The ionic formula of aluminium bromide is AlBr3. This formula explicitly indicates the presence of one aluminium ion (Al^3+) and three bromide ions (Br^-) in each molecule of aluminium bromide. It reflects the balanced charges between the cations and anions in the compound, resulting in an overall neutral electrical charge.
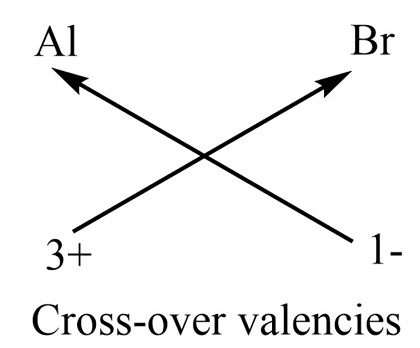
Aluminium Bromide Formula Chemical Equation
The chemical equation representing the formation of aluminium bromide formula can be described as:
Al + 3Br2 → 2AlBr3
In this equation, solid aluminium (Al) reacts with bromine gas (Br2) to produce solid aluminium bromide (AlBr3). The balanced equation highlights the fact that three moles of bromine gas are required to react with one mole of aluminium to produce two moles of aluminium bromide. The equation also demonstrates the stoichiometry of the reaction.
Understanding the chemical formula of aluminium bromide and its ionic nature is crucial in various chemical and industrial processes, as this compound has applications in areas such as catalysis, organic synthesis, and as a Lewis acid in chemical reactions. Its properties are closely related to the compound's specific composition of aluminium cations and bromide anions.
Physical Properties of Aluminium Bromide
The compound aluminium bromide appears as a white to yellowish-red lumpy solid. The aluminium bromide solution appears as a light-coloured liquid. Furthermore, aluminium bromide has a strong odour. Additionally, this compound is extremely corrosive to the skin and eyes. Its boiling point is 255 °C, while its melting point is 97.5 °C.
In addition to being soluble in many organic solvents, aluminium bromide fumes strongly in the air due to its nature, and these solvents include nitrobenzene, toluene, xylene, benzene, and simple hydrocarbons. In comparison to trialkylaminums, aluminum bromide is less sensitive to oxidation. Furthermore, one can heat this compound to its decomposition point. When aluminium bromide decomposes, it emits toxic fumes known as hydrogen bromide.
Chemical Properties of Aluminium Bromide
The predominant variation of aluminium bromide is Al2Br6, which has the ability to dissociate and form a powerful Lewis acid known as AlBr3. Additionally, there is an inclination for Al2Br6 to combine with itself, forming larger structures. This phenomenon is commonly observed in heavier main group halides, which tend to exist as larger aggregates than their empirical formula suggests. Unlike heavier main group halides, such as boron tribromide, this tendency is not seen in lighter ones due to the smaller size of their central atom.
Water hydrolyzes Al2Br6 in accordance with its characteristics of Lewis acid. Furthermore, this hydrolyzation takes place with HBr evolution and the formation of Al-OH-Br species. Additionally, it reacts rapidly with alcohols and carboxylic acids.
In addition to its less vigorous reaction than water, Al2Br6 forms adducts with simple Lewis bases. Additionally, aluminium tribromide forms carbon tetrachloride when it reacts with carbon tetrachloride at 100 °C.
| Related Links | |
| Ascorbic Acid formula | Acetaldehyde Formula |
| Butane Formula | acetonitrile formula |
Aluminium Bromide Formula FAQs
What is AlBr3 called?
What is the formation of aluminum bromide?
What is the chemical name for Al2Br3?
What is the use of aluminum bromide?










