
Potassium Hydroxide Formula: Potassium (K) is an element and its atomic number is 19. It is very soft, silvery-white metal that can be easily cut with a knife due to its malleability. When exposed to atmospheric oxygen, potassium rapidly forms flaky white potassium peroxide in a matter of seconds.
Hydroxide is a diatomic anion represented by the chemical formula OH⁻. Hydroxide anion consists of an oxygen and a hydrogen atom, bonded together by a single covalent bond and carrying a negative electric charge. It is typically a minor component in water, it plays important roles as a base,a nucleophile, a ligand, and a catalyst.
Potassium hydroxide is represented as KOH,It is an inorganic compound. It is commonly known as caustic potash. Together with sodium hydroxide (NaOH), KOH is a potent, solid base. Its chemical properties, marked by its corrosive nature and reactivity with acids, make it valuable for numerous industrial and specialized applications.
Potassium Hydroxide Formula
The potassium hydroxide formula is KOH, and its molar mass is 56.11 g/mol. KOH is composed of an ionic bond, linking the potassium metal cation with the hydroxyl anion.
Potassium Hydroxide Formula Name
The potassium hydroxide formula is KOH, and it is commonly known as "potassium hydroxide."
Potassium Hydroxide Formula Structure
Furthermore, solid KOH is found in a rhombohedral translucent structure, similar to that of sodium chloride.
The structure of potassium hydroxide is such that at high temperatures, solid KOH adopts a crystalline structure resembling NaCl. The hydroxyl group (OH) may be randomly or rapidly disordered, giving the OH- group a spherical anion shape with a radius of 1.53 Å, falling between the sizes of Cl and F ions.
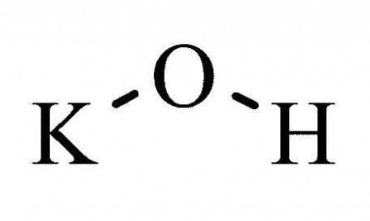 At room temperature, the OH- group and the Climate around K+ ions are distorted, with K+-OH- distances ranging from 2.69 to 3.15 Å, depending on the orientation of the OH group. KOH can form several translucent hydrates, including the monohydrate KOH·H2O, the dihydrate KOH·2H
2
O, and the tetrahydrate KOH·4H
2
O.
At room temperature, the OH- group and the Climate around K+ ions are distorted, with K+-OH- distances ranging from 2.69 to 3.15 Å, depending on the orientation of the OH group. KOH can form several translucent hydrates, including the monohydrate KOH·H2O, the dihydrate KOH·2H
2
O, and the tetrahydrate KOH·4H
2
O.
Potassium Hydroxide Formula and Valency
The potassium hydroxide formula is KOH. In this formula:
K represents the element potassium, which has a valency of +1.
O represents the element oxygen, which typically has a valency of -2.
H represents the element hydrogen, which usually has a valency of +1.
The combination of these elements and their respective valencies results in the formula KOH for potassium hydroxide.

Potassium Hydroxide Formula Properties
Potassium hydroxide comes in clear pellets. They get cheaper when exposed to the air because KOH absorbs moisture, so it often contains varying amounts of water (H2O). When you dissolve it in water. then it releases a lot of heat. These concentrated watery solutions are usually called potassium lye. Surprisingly, even at higher temperatures, solid KOH doesn't easily lose its moisture.
- Chemical Formula: KOH
- Molar Mass: 56.11 grams per mole
- Density: 2.12 grams per cubic centimeter
- Melting Point: 360 degrees Celsius
- Boiling Point: 1,327 degrees Celsius
Preparation of Potassium Hydroxide(KOH)
To produce potassium hydroxide, there use of a method called the "chloralkali process." In this process, they pass electricity through potassium chloride solutions. This process yields potassium hydroxide along with chlorine gas as a by-product:
2 KCl + 2 H 2 O → 2 KOH + C l2 + H 2 .Uses of Potassium Hydroxide
Salt Preparation: KOH is a strong alkali that easily reacts with various acids. This alkaline reaction is used to produce potassium salts, which find applications in diverse industries.
Acid Neutralization: As a soluble base, potassium hydroxide is utilized to neutralize acidity and adjust the pH of solutions. In chemical analysis, it plays a important role in titrating acids to determine their concentration.
Soap Production: Potassium hydroxide is involved in the saponification process, where it reacts with oils and fats under heat, leading to the production of potassium soaps. This reaction is essential in soap manufacturing.
Liquid Fertilizer Production: Making liquid fertilizers often involves using potassium hydroxide.
| Related Links | |
| Potassium Fluoride Formula | Potassium Chlorate Formula |
| Potassium Bromate Formula | Gold Formula |
Potassium Hydroxide Formula FAQs
What is the formula of potassium hydroxide?
What is the molar mass of KOH?
How is KOH structured at high temperatures?
What is the chemical formula for hydroxide ion?










