
Aluminium Oxide Formula : Aluminium oxide or aluminium (III) oxide formula is Al2O3. Aluminium oxide consists of aluminium and oxygen. Among several aluminium oxides, it is the most prevalent and is specifically recognized as aluminium oxide. It is usually called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications.
Naturally, aluminium oxide exists in its crystalline polymorphic phase known as α-Al 2 O 3 , which is found in the mineral corundum. Various forms of corundum give rise to precious gemstones such as ruby and sapphire. Al 2 O 3 plays a crucial role in the production of aluminium metal due to its hardness, and it serves as an abrasive material. Additionally, its high melting point makes it invaluable as a refractory substance.
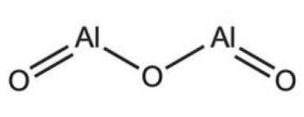
Aluminium Oxide Formula Structure
Aluminium Oxide Formula is Al 2 O 3 . The structure of aluminum oxide is created by the bonding of two aluminum atoms with two oxygen atoms each, resulting in a total of three oxygen atoms.
Aluminium Oxide Formula Physical Properties
Aluminium Oxide Formula is Al 2 O 3 ..
Aluminium oxide is a whitish solid.
Aluminium oxide has a relatively high melting point 2345K.
Aluminium oxide has a boiling point of 3250K.
Aluminium oxide has a density of 3.987g/cm3.
Aluminium oxide is insoluble in water and as well as in all other solvents.
Even aluminium oxide is an electrical insulator.
Aluminium oxide exhibits thermal conductivity in ceramic material.
Aluminium Oxide Formula Chemical Properties
Aluminium Oxide Formula is Al 2 O 3 . when aluminium oxide reacts with sodium hydroxide. Then Sodium aluminate and water are produced and This reaction occurs at temperatures ranging from 900 to 1100°C. This reaction, in which aluminium oxide is used as an acid and produces salt and water.
Al 2 O 3 + 2NaOH → 2NaAlO 2 + H 2 O
Metal oxides are generally basic in nature, Even aluminium oxide is amphoteric. As an effect , it is used as both an acid and a base. Belwo case, it does not act as an acid but acts as a base.
Al 2 O 3 + H 2 SO 4 → Al 2 (SO 4)3 + H 2 O
This is commonly known as a neutralization reaction.
Due to oxide ions of Aluminium oxide. It reacts with acids similarly to sodium or magnesium oxides. Aluminium chloride solution is produced when aluminium oxide reacts with hot dilute hydrochloric acid.
Al 2 O 3 + 6HCl → 2AlCl 3 + 3H 2 O
Aluminium Oxide Uses
Aluminum oxide serves multiple essential roles across various industries:
Its exceptional hardness and strength make aluminum oxide a popular choice as an abrasive, often replacing industrial diamonds in the production of sandpaper, cutting tools, and various other products.
Aluminum oxide serves as a catalyst in numerous industrial processes, such as the Claus process, which converts hydrogen sulfide waste into elemental sulfur.
It is used to remove water from gas streams.
Aluminum oxide flakes are integrated into paints to create a reflective or decorative appearance.
It is a common ingredient in cosmetics, being used in products like sunscreen, lipstick, blush, and nail polish.
In the manufacturing of glass, aluminum oxide plays a significant role, particularly in aluminosilicate glass, where it typically comprises 5-10% of the composition.
Transparent aluminium oxide finds application in sodium vapour lamps and compact fluorescent lights.
It functions as an electrical insulator in integrated circuits.
It finds extensive use as a ceramic material.
Aluminum oxide is used as a plastic filler.
Aluminium oxide formula is Al2O3. It is a versatile compound with a robust structure, high melting point and Aluminium oxide ability to act as both an acid and a base. It is used in abrasives, catalysts, cosmetics, and various industrial applications, playing a crucial role in a wide range of products and industries. Its diverse properties make it an essential material in modern science and technology.
Enroll Online Course of Class 9 Neev Fastrack 2024 and Class 10 Udaan Fastrack 2024 to enhance your chemistry knowledge. and build a strong foundation.
| Related Links | |
| Copper I Chloride Formula | Ammonium Hydroxide Formula |
| Copper (II) Chloride Formula | Copper Sulfate Formula |
Aluminium Oxide Formula FAQs
What is the chemical formula of aluminium oxide?
What is the primary structure of aluminium oxide?
What are the key physical properties of aluminium oxide?
What are the main uses of aluminium oxide?










