The aluminium sulfide formula, also known as aluminium sulfide, is a chemical compound composed of aluminium and sulfur. Its formula and various aspects related to it are essential for understanding its properties and behaviour.
Aluminium Sulfide Formula
The aluminium sulfide formula is Al₂S₃. It consists of two aluminium (Al) atoms and three sulfur (S) atoms, which combine in a specific ratio to form this compound.
Among its many forms, aluminium sulfide has six crystalline forms. Aluminium sulfide is synthesized by thermitic reactions between aluminium powders and sulfur.
In addition to being known as dialuminium trisulfide, aluminium sulfide is an inorganic compound that is often used as a raw material during the synthesis of hydrogen sulfide. Additionally, it is moderately water-soluble and acid-soluble. Also, aluminium sulfide has a fascinating structural history and exists in several forms. It is also sensitive to water, hydrolyzing to hydrated aluminium oxides.
The term "aluminium sulphide" is a common alternative spelling for aluminium sulfide. It refers to the same compound, and its chemical formula remains Al₂S₃.
Also Read: Propionic Acid Formula
Aluminum Sulfide Formula Explained
The aluminium sulfide formula Al₂S₃ represents the stoichiometry of aluminium sulfide, indicating that for every two atoms of aluminium, there are three sulfur atoms in the compound. This balanced combination provides insight into the compound's composition.
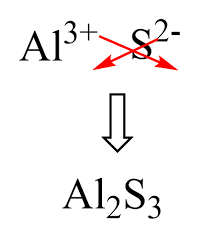
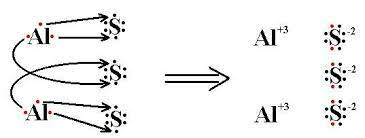
Al2S3 is the formula for aluminium sulfide. It is possible for this compound to exist as a hydrate form. Furthermore, in such a state, water molecules hydrate it. This compound is formed by combining two cations and three anions Al3+ into Al2S3. Therefore, its formula is Al2S3. The crystal structure of aluminium sulfide is hexagonal, tetragonal, and triangular.
Aluminium Sulphide Formula by Criss-Cross Method
The criss-cross method is a simple technique for determining the chemical formula of ionic compounds. In the case of aluminium sulfide formula , the charges on aluminium and sulfur ions are +3 and -2, respectively. By criss-crossing these charges, you arrive at the formula Al₂S₃, which reflects the neutral compound formed by the combination of these ions.
Also Read: Nickel Chloride Formula
The charges on the individual ions in aluminium sulfide are essential for understanding its formula. Aluminium (Al) has a charge of +3, while sulfur (S) has a charge of -2. When these ions combine, the charges balance out to form the neutral compound Al₂S₃.
Aluminum Sulfide Formula State
Aluminium sulfide is a crystalline solid with a state that depends on temperature and pressure. It typically exists as a white or off-white solid at room temperature and pressure. However, it can undergo changes in state under varying conditions.
The formula unit of aluminium sulfide, Al₂S₃, represents the smallest whole-number ratio of atoms in the compound. It signifies that there are two aluminium atoms and three sulfur atoms in one unit of aluminium sulfide.
Aluminum Sulfide Formula Reaction
Aluminium sulfide can participate in various chemical reactions. For example, when exposed to water, it reacts to produce aluminium hydroxide and hydrogen sulfide gas:
Al₂S₃ + 6H₂O → 2Al(OH)₃ + 3H₂S
This reaction showcases the compound's reactivity with water.
Aluminum Sulfide Formula of Compound
The aluminium Sulfide formula Al₂S₃ provides a concise representation of the compound's composition, clearly indicating the number of aluminium and sulfur atoms in each molecule of aluminium sulfide.
Aluminium Sulfide Properties
Aluminium sulfide, with the chemical formula Al₂S₃, is a compound with distinct properties that make it valuable in various applications and chemical processes. Here, we will explore some of its essential properties:
| Properties of Aluminium Sulfide | |
| Name | Aluminium Sulfide |
| Appearance | Gray solid with rotten egg smell |
| Chemical Formula | Al 2 S 3 |
| Melting Point | 1,100 °C |
| Boiling Point | 1, 500 °C |
| Density | 2.02 g/cm 3 |
| Molar Mass | 150.158 g/mol |
| Solubility in Water | Hydrolyses to release H 2 S |
Also Read: Lead Iodide Formula
Uses Of Aluminium Sulfide
- Hydrogen sulfide is prepared from aluminium sulfide.
- Chemical industries primarily use it.
- Cathodes are made from it.
- Nano-network structures are produced using it.
- The molecule has a significant impact on the environment.
| Related Links | |
| Magnesium Iodide formula | Aluminium chloride formula |
| Inorganic Compound | Aluminium fluoride formula |
Aluminium Sulfide Formula FAQs
How do you write aluminum sulfide?
Is the formula Al3S2 for aluminium sulfide correct? Why or why not?
How is aluminium sulfide used?
What is the symbol for aluminium sulfide?