
Plaster of Paris, often referred to as P.O.P., is a versatile and widely used material with various applications in the fields of construction, art, and medicine.
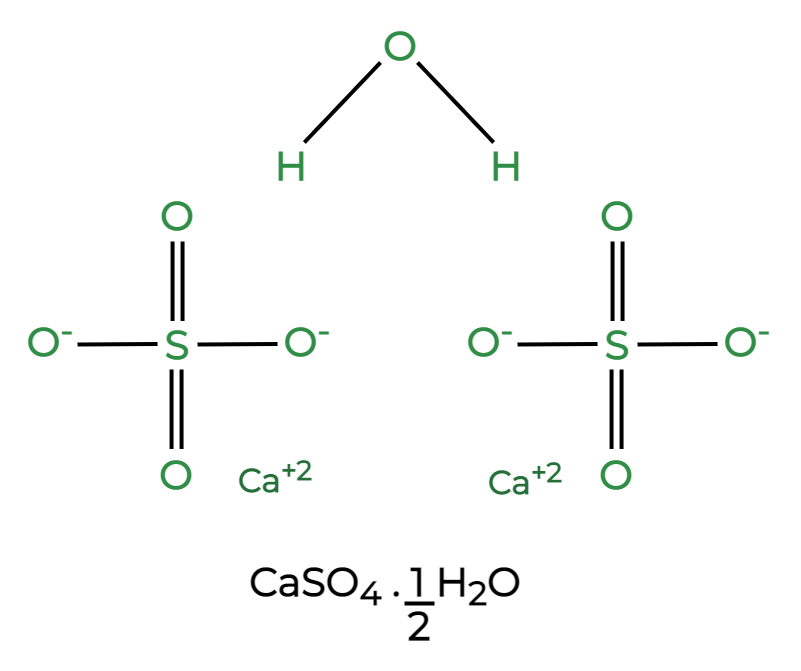
The chemical formula for the plaster of Paris is CaSO₄·0.5H₂O. It is composed of calcium sulfate hemihydrate, where one-half molecule of water is chemically combined with one molecule of calcium sulfate. This formula signifies its unique structure, allowing it to be easily rehydrated.
Plaster of Paris Formula
The formula for plaster of Paris is synonymous with its chemical formula, which is CaSO₄·0.5H₂O. This compound is created through the partial dehydration of gypsum (calcium sulfate dihydrate), resulting in a powder that can be mixed with water for various applications.
Also Read: Lactic Acid Formula
Types of Plaster of Paris
Three types of plaster in Paris are widely used:
Gypsum Plaster: Gypsum plaster, also known as Plaster of Paris, involves heating it to 300 °F. When gypsum is heated over 392 °F, anhydrite is formed. Plaster of Paris hardens very quickly when added to water. Dry gypsum plaster powder turns into gypsum when combined with water.
Cement Plaster: A final coat of gypsum plaster over the cement plaster is often applied to provide a smooth surface. Cement plaster comprises suitable plaster, Portland cement, sand, and water.
Lime Plaster: It is a compound made of sand, calcium hydroxide, and various inert fillers. Quick lime is created by heating limestone, while slaked lime is created by mixing water with quick lime.
Clay Plaster: Throughout history, clay plaster has been used to make the interiors of houses with sand, water, clay, and plant fibers added for strength. This plaster had been used since ancient times.
Heat Resistant Plaster: Portland cement, for example, is a heat-resistant plaster used for coating walls, ceilings, chimneys, etc.
Also Read: Galactose Formula
Plaster of Paris Properties
- Rehydration: It has the remarkable property of being able to rehydrate when mixed with water. This property makes it valuable for setting broken bones and creating molds for sculptures.
- Ease of Use: It can be easily mixed with water to form a workable paste that hardens rapidly, making it convenient for a variety of applications.
- Hardening: When it is mixed with water, it hardens through a process called crystallization, forming a solid and durable structure.
- White Color: It has a naturally white color, which makes it ideal for art and construction projects where a clean and bright finish is desired.
Also Read: Potassium Acetate formula
Plaster of Paris Uses
- Medical Applications: It is commonly used in orthopedics for creating casts to immobilize and support fractured or injured bones.
- Art and Sculpture: It is widely used by artists and sculptors to create intricate sculptures and molds due to its ease of use and fine detailing capabilities.
- Construction and Repair: It is used for repairing and finishing interior walls and ceilings. It is also employed in architectural detailing.
- Dental Industry: In the dental field, it is used to create moulds for teeth and dental appliances.
- Educational Models: It is utilized for creating educational models and dioramas in schools and museums.
How is Plaster of Paris Made?
The production of plaster of Paris involves the following steps:
- Mining Gypsum: Gypsum, a naturally occurring mineral, is first mined and processed to remove impurities.
- Calcination: The gypsum is then subjected to heat to partially dehydrate it, converting it into calcium sulfate hemihydrate, which is the main component of plaster of Paris.
- Grinding: The hemihydrate is ground into a fine powder, resulting in plaster of Paris.
- Packaging: The final product is packaged and distributed for various applications, with instructions for rehydration provided as needed
| Related Links | |
| Chromium III Chloride Formula | Cinnamic Acid Formula |
| Ammonia Carbonate Formula | Trichloroacetic Acid formula |
Plaster of Paris FAQs
Which plaster of Paris is best?
What are the three types of plaster?
What type of plaster is plaster of Paris?
Are there different types of plaster?










