Details Of h2O Molecules
Cellular Respiration of Class 11
H2O molecules formed
(i) In Glycolysis : (total 4H2O molecules are formed)
(a) 2H2O at step 9 during conversion of 2-PGA to 2-PEP
(b) 2H2O are formed on oxidation of 2NADH through ETS in mitochondria
(ii) In Mitochondria (Total 12H2O molecules are formed)
- 2H2O are formed during oxidation of 2NADH formed in transition reaction. It occurs through ETS.
- 2H2O are formed in Krebs’ cycle at step 2 during formation of cis aconitic acid.
- 8H2O are formed in Kreb’s cycle during the oxidation of 6NADH and 2FADH2 formed at setp 4,6,8 and 10. It occurs through ETS.
Thus out of 12, 10 H2O molecules are formed in Krebs’ cycle.
H2O Molecules consumed
Out of 16 molecules of H2O, 10 are consumed as follows :
(a) 2H2O are consumed in glycolysis at setp 5 during the formation of 1,3 - bisphosphoglyceraldehyde.
(b) 8H2O are consumed in Krebs’ cycle (2 in the conversion of OAA to citric acid at step 1; 2 each at step 3,7 and 9).
Thus net gain of H2O is 16 – 10 = 6
Terminal (Biological) oxidation or oxidation of reduced Coenzymes
The entire process of terminal oxidation includes 2 steps:
- Electron Transport System
- Oxidative Phosphorylation
Electron Transport System : Each oxidative step involves release of a pair of hydrogen atoms (2H) which dissociate into 2H+ (Protons/Hydrogen ion) and 2e– (electrons); 2H 2H++ 2e–. These protons and electrons do not combine with oxygen directly but pass through a series of coenzymes (hydrogen carrier) and cytochromes (electron carriers) which are associated with proton transport enzymes in oxysomes (F0 – F1 particles), before reacting with oxygen to form metabolic water.
The series of coenzymes, cytochromes and associated enzymes forms Electron Transport Chain (ETC)/Electron Transport System (ETS) /respiratory chain/cytochrome chain.
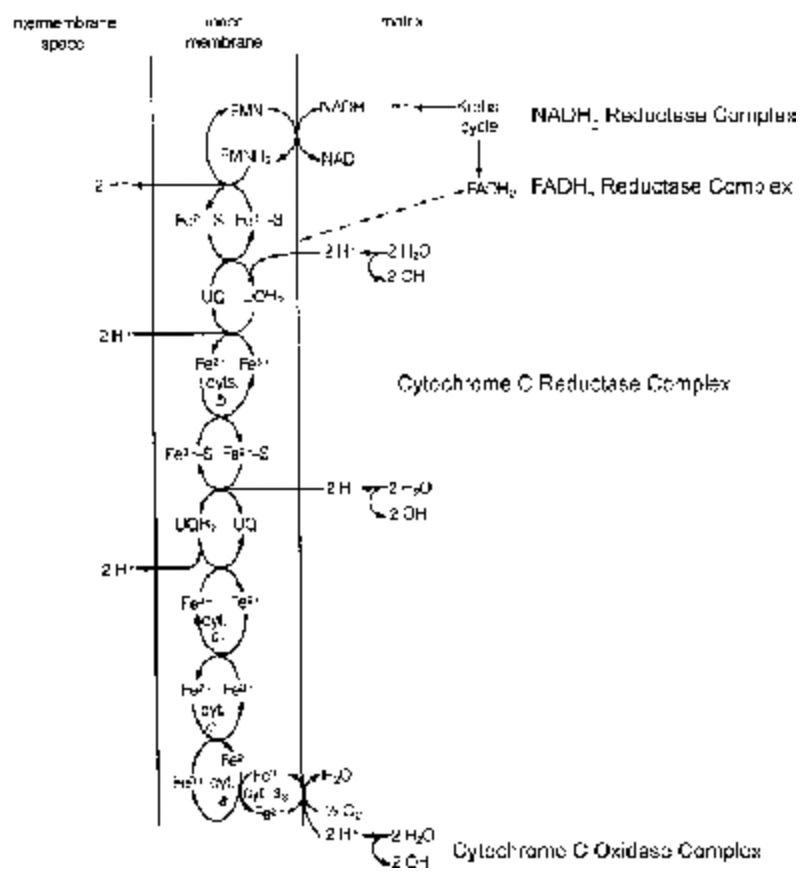
Oxidative Phosphorylation:
It is a mode of synthesis of ATP from ADP and inorganic phosphate with the help of energy released by electrons during their passage over electron transport chain when reduced coenzymes produced in respiration are oxidised at the time of terminal oxidation in mitochondria.
ATP synthesis is an endothermic (endergonic), oxidative process that occurs on the inner membrane including the cristae of mitochondria in eukaryotes and innerside of cell membrane/mesosome in prokaryotes. In photosynthetic eukaryotes, mitochondria are the major site of ATP production in the dark but in day light, chloroplasts produce most of the ATP.
Several hypotheses have been proposed to explain the mechanism of ATP synthesis both in chloroplast and mitochondria. Most accepted one is that of Mitchell’s chemiosmotic coupling hypothesis (Peter Mitchell, 1961, Nobel Prize in 1978). According to this hypothesis:
- Inner membrane of mitochondria act as a transducer and convert the energy due to electro chemical gradient into chemical energy of ATP.
- Inner membrane of mitochonrdia is impermeable to NADH, ATP, Protons (H+) and OH– ions.
- ATP synthesis and electron transpost are coupled through proton translocation.
This process of ATP synthesis is called chemiosmosis because it entails (i) chemical reaction (formation of ATP from ADP) and (ii) active transport of electron across the membrane (=osmosis). ATP synthesis involves 2 steps.
Creation of Proton gradient / Proton motive force: During the passage of two electrons from one electron carrier (NADH) to another on ETC, enough energy is liberated that pumps out three pairs of protons (Hydrogen atoms H+) from inner chamber (matrix) to outer chamber (inter membrane space) of mitochondrion. As a result of this, the concentration of protons (H+) increase in intermembrane space and its pH decreases. This sets up a proton gradient and electrochemical potential (both collectively called Proton motive force) across the inner membrane. This force activates ATPase in F1 subunit of F0–F1 particles for ATP synthesis.
Proton Flow : Due to proton motive force, the protons flow back by active transport from outer chamber to inner chamber through three specific coupling sites (proton channels) in the region of F0 of F0 - F1 particles (=oxysomes) are present. Inner membrane of mitochondria is impermeable to protons. OH–, Cl–, NADH and ATP and the protons (H+) can move only through proton channels present in F0 subunit of F0 - F1 particles embedded in mitochondria. There are 104 to 105 such particles per mitochondrion. F1 subunit is called head and has ATPase (ATP synthetase) for the synthesis of ATP. F0 subunit is called base and has 3 Proton channels. It is embedded in inner membrane.
Energy is released during this back flow (active diffusion) of protons from outer chamber to inner chamber of mitochondria. This energy activates ATPase in head (F1) region to synthesize ATP from ADP and iP. Na+, K+ are required. Thus synthesis of ATP is energy requiring (endergonic) process. One ATP is produced for every two protons passing through F0-F1 particles. Passage of 2e– from NADH over ETC to O2 transfer 3 pairs of protons to outer chamber. Return of these 3 pairs of electrons back into inner chamber will produce 3 ATP
Production of ATP from NADH + H+ produced in cytoplasm during Glycolysis
In Eukaryotes out of 38 ATP produced per glucose molecule, 2 are used in bringing 2 molecules of NADH + H+ formed in glycolysis from cytosol to mitochondria for oxidation through ETS (Glycerol - phosphate shuttle). Thus net gain is 36 ATP. Out of these 36 ATP, 2 are formed outside and 34 inside the mitochondrion. In Prokaryotes the value remains to be 38 as they don’t need to spend 2 ATP to transport 2NADH+H+ to mitochondrion because they do not have mitichondrion. Actually NADH+H+ cannot enter the mitochondria to pass e– to the ETS because the inner mitochondrial membrane is impermeable to NADH and H+ (Protons). Therefore e– carried by NADH+H+ are transferred to other substance that act as electron shuttle between exterior and interior of Mitochondria.
The shuttle acts as electron carrier and picks up 2e– from the hydrogen of NADH present in the cytoplasm, transfers them across the membrane and pass on to electron carriers inside the mitochondrion. Two types of shuttles are known which are located in the mitochondrial membrane.
(1) Malate - Aspartate Shuttle: It is a more efficient system, present in the heart, liver and kidney cells. It transfers electrons from NADH in cytoplasm to NAD inside mitochondrion. In this shuttle system there is no loss of any ATP and net ATP production remains to be 38 per glucose molecule.
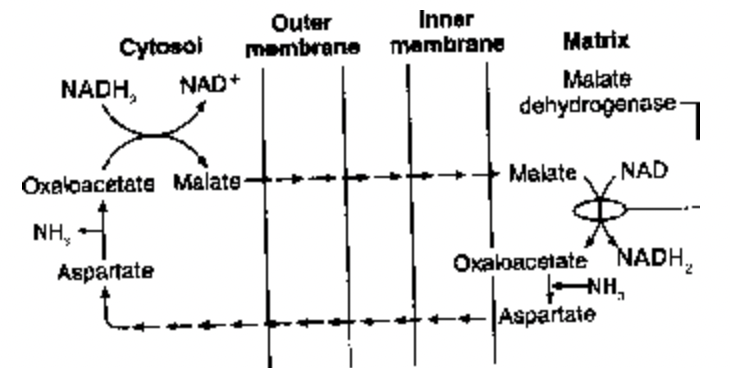
Fig. Malate-aspartate shuttle
(2) Glycerol - Phosphate Shuttle : It is less efficient. It transfers electrons from NADH+H+ in cytoplasm to FAD in mitochondrion and reduce it to FADH2. Only 2ATP instead of 3 ATP are formed for each molecule of FADH2 oxidised. When electrons from NADH2 are carried by glycerol phosphate shuttle into mitochondrion, there is a loss of 1 ATP per molecule of NADH + H+ oxidation through this shuttle in ETS. Thus, net ATP gain is 36 per glucose molecule.
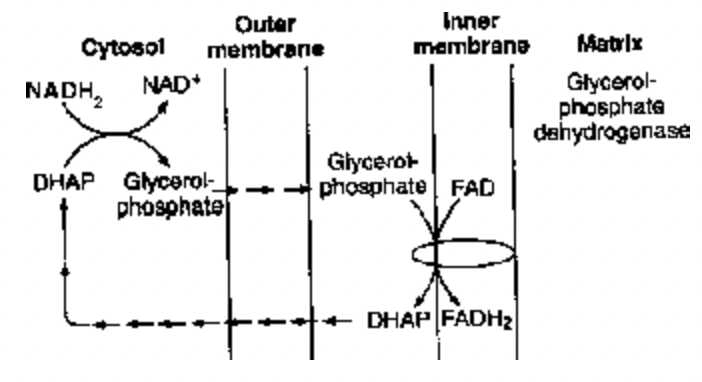
Fig. Glycerol-Phosphate Shuttle









