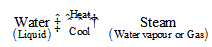Classification of Changes
Changes Around Us of Class 6
Classification of changes
A substance undergoes a change to form a ‘new substance’ only when certain agents like heat, light, electricity or force, etc., are applied to it. Now, if the agent causing the change is removed, then in some cases the ‘new substance’ undergoes a change in the reverse direction to form the ‘original substance’. And we say that the change can be reversed or that the change is reversible. If, however, the ‘new substance’ formed does not undergo reverse change to form the ‘original substance’, then we say that the change cannot be reversed or that the change is irreversible. In reality, some of the changes can be reversed whereas other changes cannot be reversed. So, all the changes around us can be classified into two groups:
1. Reversible changes (Changes which can be reversed), and
2. Irreversible changes (Changes which cannot be reversed).
We will now discuss reversible changes and irreversible changes around us in detail, one by one. Let us satart with reversible changes.
Reversible changes
A change which can be reversed to form the ‘original substance’ is called a reversible change. This will become clear from the following example. Ice is a solid substance. When we heat ice, it melts to form liquid water. A change from solid to liquid takes place during the melting of ice. Now, if we cool the water (formed from ice) by keeping in the freezer of a refrigerator, it again changes into solid ice. So, the change from ice to water, by heating, has been reversed by cooling. Thus, the melting of ice (to form water) is a reversible change. This reversible change can be represented as follows:
When we keep some ice at room temperature, it appears to melt on its own to form water. In this case, the heat required to molt ice is supplied by the surrounding air (which is at a higher temperature than ice).
There are a very large number of reversible changes around us. Some of the example of reversible changes (or changes which can be reversed) are: Melting of ice; Boiling of water; Melting of wax; Stretching of rubber band; Stretching of spring; Inflating a balloon; Ironing of clothes; Folding of paper; Rolling a chapatti(roti) From dough Dissolving salt in water; Dissolving sugar in water; Knitting of sweater (Woollen yarn to knitted sweater); Melting of ice candy; Melting of ice cream (Solid ice cream to molten ice cream); Drying of clothes (Wet clothes to dry clothes); Heating of milk (Cold milk to hot milk); Expansion of metals on heating; Straight wire to coiled wire; Melting of coal tar; Folding of dress (trousers, etc.) to pass through waterlogged area; Moulding of wet clay into pot on a potter’s wheel; and Softening of iron on heating to red-hot stage. We will discuss some of these changes in somewhat detail.
When we boil water by heating, then its changes into steam. Now, if we cool the steam, then water is formed again. So, the changing of water into steam has been reversed by cooling. Thus, the boiling of water (or evaporation of water) is a reversible change. This can be shown as follows:
When ice changes into water, then there is a change from solid state to liquid state. And when water changes into steam, then there is a change from liquid state gaseous state. So, in general we can say that: Change of state is a reversible change.
If we stretch a rubber band with the force of our hands, it undergoes a change and its length increases. But on releasing the force, the rubber band comes back to its original length. This means that the change which occurred in rubber band on stretching, has been reversed on releasing. So, the stretching of rubber band is a reversible change. Similarly, the stretching of a spring is also a reversible change.
Take some salt and dissolve it in water taken in a breaker. A salt solution is formed. A change has occurred in salt during the formation of salt solution (because salt has disappeared into water). This change can however, be reversed as follows: Keep the beaker containing salt solution over a burner and evaporate it. On evaporation, water is eliminated and salt is left behind. This, dissolving salt in water is a reversible change.
Coal tar is a black, solid material which is used in making and repairing roads. When coal tar is heated, it melts to form a thick black liquid. The melting of coal tar on heating, is a reversible change. This is because when hot, molten coal tar gets cooled, it solidifies again.
A blacksmith (lohar) changes the pieces of iron into different tools like hammer, axe, spade, etc. This is done as follows: The piece of iron metal is heated in a furnace till it becomes red-hot. At red-hot stage, the piece of iron becomes soft. The red-hot piece of iron (being soft) can be hammered easily into the desired shape to make a tool. And when the hot iron tool is cooled, it becomes hard again. Thus, the softening of iron on heating to red-hot stage is a reversible change. This is because when red-hot iron is cooled, it becomes hard again.
In order to pass through a waterlogged area (as during rains), we usually shorten the length of our dress (like trousers, etc.) temporarily, by folding it up. The folding up of a dress (trousers, etc.) to pass through a waterlogged area is a reversible change. This is because it can be reversed by unfolding the dress.
Expansion (on Heating) is a Reversible Change
When an object is heated, it increases in size. The increase in size on heating, is called expansion. The expansion of an object on heating, is a reversible change. This is because when the hot object is cooled, it decreases in size and comes back to the original size. The decrease in of an object on cooling, is called contraction. Thus, in most simple terms: Expansion mean increase in size and contraction means decrease in size. Expansion occurs on heating whereas contraction occurs on cooling. The reversible change of expansion used.
(i) In fixing an iron rim on the wooden wheel of a cart, and
(ii) In fixing the iron blade of a digging tool (like a spade) to a wooden handle.
1. Fixing or iron Rim to the wooden wheel of a cart
All of us have seen the wooden wheels of bullock carts and horse carts (tongas) having thin iron rims (or thin iron types) around them. The iron rims are fitted around wooden wheels by the process of expansion on heating (followed by contraction on cooling). This is done as follows.
The iron rim is made slightly smaller than the wooden wheel (around which it is to be fitted). The iron rim is heated uniformly by making a fire due to which it expands and becomes somewhat bigger in size. Being bigger in size, the hot iron rim is easily put around the wooden wheel. Cold water is then poured over the hot rim to cool it. On cooling, the hot iron rim contracts (becomes slightly smaller in size) and fits tightly on the wooden handle.
Irreversible Changes
A change which cannot be reversed to form the original substance (or substances) is called an irreversible change. This will become clear from the following example. If we burn a piece of paper, it changes into ash and smoke. Now, we cannot combine the ash and smoke to form the original piece of paper. So, the burning of paper is a change which cannot be reversed. In other words, the burning of paper is an irreversible change.
There are a very large number of irreversible changes us. Some of the examples of irreversible changes (or changes which cannot be reversed) are: Burning of paper, Burning of fuels (like Wood, Coal and LPG); Formation of curd from milk; Cooking of food; Rusting of iron ; Grinding of wheat grains into flour; Baking of chapatti(roti); Growth of a plant; Formation of flower from bud; Falling of leaves from a tree; Ripening of fruits; Ageing of man and animals; Death and decay of plants and animals; Weathering of rocks (Breaking down of rocks); Printing of paper; Souring of milk; Boiling of an egg (Raw egg to boiled egg); making cheese (paneer) from milk; Cow-dung to biogas; Sawing (cutting) of a log f wood; Breaking of a toy; Cutting of paper; Bursting a balloon; Burning of wax (in the form of candle); Burning of incense stick (agarbatti); Setting of Plaster of (POP) on mixing water; Setting of cement on mixing water; Growth of nails on fingers; Making painting on a drawing sheet; and Baking a clay pot in a oven. We will discuss some these change in somewhat detail.
Curd is made from milk. This is done as follows: A very small quantity of previously made curd is added to warm milk. The milk is then stirred and kept aside for a few hours at a warm place. During this time, milk changes into curd. This curd, however, cannot be changed back into milk by an means. So, the formation of curd from milk is an irreversible change (which cannot be reversed).
When wheat is ground, it is converted into flour. The flour cannot be converted back into wheat grains. So, the grinding of wheat to form flour is an irreversible change (which cannot be reversed). When we heat raw food materials, we get cooked food. The cooked food cannot be converted back into raw food materials. So, the cooking of food is an irreversible change (which cannot be reversed). A young man ages and ultimately becomes an old man. But we cannot change an old man back into a young man. So, the ageing of man is an irreversible change (which cannot be reversed).
If we drop a toy accidently, it breaks into a number of pieces. We cannot get back original toy again from the broken pieces. This means that the change which takes place during the breaking of a toy, cannot be reversed. In other words, the breaking of a toy is an irreversible change. When we draw and paint a picture on a drawing sheet, there is a change in the drawing sheet. We cannot convert the painted drawing sheet back into the original, blank drawing sheet. So, the making of painting on a drawing sheet is an irreversible change (which cannot be reversed).
Plaster of Paris (POP) is a white, powdery substance which immediately sets to a hard mass on adding water to it. In hospitals, a thick coat of the paste of plaster of Paris is applied over the bandage on the fractured bone of a person. POP sets and becomes hard on drying and keeps the fractured bone in place to get joined properly. The change which occurs in POP during setting cannot be reversed. Thus, the setting of plaster of Paris on maxing water is an irreversible change.
When water is added to cement, it sets into a hard mass after sometime. If a bag of cement lying in the open gets wet due to rain during the night, some change occur in cement due to which it gradually sets into a hard mass. Even if we keep the wet cement in bright sunshine the next day, the changes which have taken place in cement on getting wet, cannot be reversed. Thus, the setting of cement on mixing with water is an irreversible change.
If we light an incense stick (agarbatti) with a burning a burning matchstick, we find the after some time, the whole incense stick burns away. During the burning of incense stick, some pleasant smelling gases are produced (which go into the air), and ash is left behind. We cannot recombine the gases and the ash to get back the original incense stick. So, this is a change which cannot be reversed. Thus, the burning of an incense stick is an irreversible change.