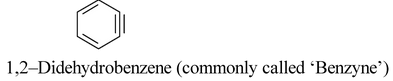Polycyclic Molecules
Nomen-Org-Compound of Class 11
Assemblies of two identical cyclic systems are named in one of two ways: (a) by placing the prefix ‘bi–’ before the name of the corresponding substituent group enclosed in parentheses, if necessary (additive operation) or (b) by placing the prefix ‘bi–’ before the name of the corresponding parent hydride enclosed in parentheses, if necessary.
For example,

A molecule that has two rings sharing a single atom is said to be spirocyclic. Such as
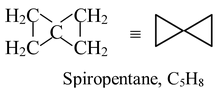
A molecule with two rings that share at least two atoms is called bicyclic.
These compound are named as derivatives of alkanes corresponding to the total number of carbons in both ring system. The term bicyclo is used as prefix together with the number of carbons in each of the three connecting arms taken in square bracket separated by full stops. For example,
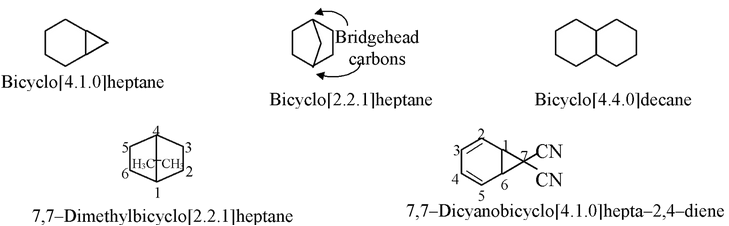
The numbering used to assign substituents or functional groups begins from the bridgehead position, proceeds along the longest arm to the other bridgehead and continues along the next longest arm. The bridgehead position selected is one that is close to the carbon bearing functional group or substituent as shown above.
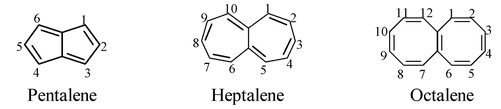
Hydrocarbons consisting of two fused identical monocyclic rings and having the maximum number of noncumulative double bonds are named by citing the numerical prefix denoting the number of carbon atoms in each ring followed by the term ‘–alene’ with elision of an ‘a’. The trivial name ‘naphthalene’ is retained.
For examples,
Hydrocarbons consisting of a monocyclic hydrocarbon with an even number of carbon atoms ortho–fused on alternate sides to benzene rings are named by citing a numerical prefix denoting the number of benzene rings followed by the term ‘–phenylene’.
For example,
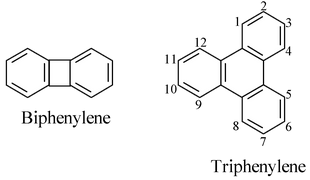
Note: Some times the presence of a double bond not implied in the parent hydride name can be indicated by a ‘didehydro–’ prefix signifying the removal of a pair of hydrogen atoms. For example,