Osmosis
Transport In Plants of Class 11
Osmosis is a special type of diffusion. Diffusion through semipermeable membrane is known as osmosis or it is process, whereby, when a solution is separated from its weaker solution by means of a semipermeable membrane, then weaker solution passes into stronger solution so as to equalize concentration on both sides.
Osmosis was discovered by Nollet.
Keeping in view that membrane is semipermeable, it allows only solvent molecules, so we can say that solvent moves from its higher conc. to lower conc.
Best way of defining osmosis is on the basis of free energy levels of solvent molecules, which is the capacity of doing work. According to this concept, solvent moves from its higher free energy level to lower free energy level (as solute molecules occupy intramolecular space of solvent molecules and hence concentration of the solvent, i.e., no. of molecules per unit volume is same in concentrated as well as dilute solution).
REVERSE OSMOSIS
It is the reverse movement of water through a semipermeable membrane from a more concentrated solution to a more dilute solution by applying external pressure on the more concentrated solution.
It is used in removing salts from saline water as well as extra purification of water.
Osmotic pressure (O.P.)
It is the pressure in atmosphere, which is required in opposite direction, so as to stop entry of water (solvent) molecules, when a semipermeable membrane separates concentrated solution from weaker solution.
Types Of Solution
Isotonic solution: When concentration of outer solution (in which cell is placed) is equal to concentration of cell sap, it is called isotonic solution.
Hypotonic solution: When concentration of outer solution (in which cell is placed) is lower than concentration of cell sap, it is called hypotonic solution.
Hypertonic solution: When concentration of outer solution (in which cell is placed) is higher than concentration of cell sap, it is called hypertonic solution.
Exosmosis and Plasmolysis
When a cell is placed in hypertonic solution water comes out from the cell into outer solution. This is exosmosis. Due to exosmosis, the protoplasm shrinks and leaves the cell wall and thus cell becomes flaccid, which is called plasmolysed cell and this phenomenon is called plasmolysis. Thus exosmosis leads to plasmolysis.
Endosmosis and Deplasmolysis
If the plasmolysed cell (flaccid cell) is placed in hypotonic solution water enters into the cell. Entry of water under influence of dilute solution is known as endosmosis. It makes the cell again turgid and this is known as deplasmolysis.
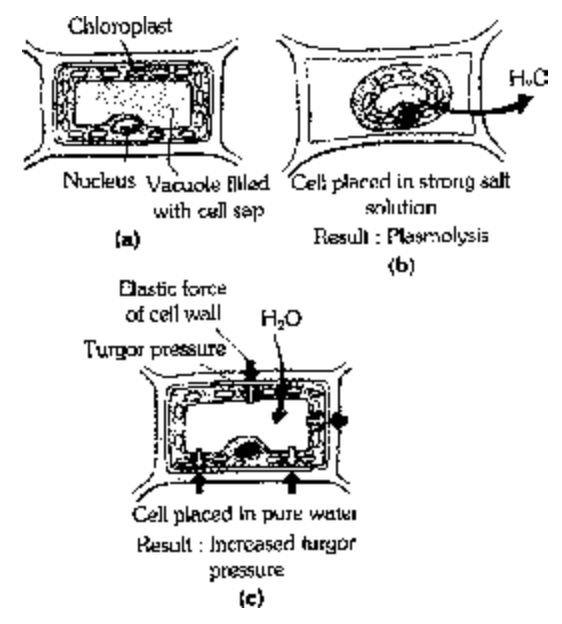
Turgor Pressure (TP)
Pressure exerted by the protoplast against the cell wall is known as turgor pressure. When a living plant cell is placed in water, it swells up due to the absorption of water by osmosis. As a result of this entry of water into the cell sap, a pressure developed in the protoplast which is exerted against the cell wall. This actual pressure is T.P. The cell wall being rigid and elastic exerts, an opposite pressure equal in magnitude to the turgor pressure. This pressure is called wall pressure (W.P.)
WP = TP
Diffusion Pressure Deficit (D.P.D.)
This term was given by Meyer.
Every liquid is having a definite diffusion pressure and diffusion pressure of pure solvent is always more than diffusion pressure of its solution.
e.g., if sugar solution is made in water, then D.P. of solution is lower than water (solvent).
The amount by which D.P. of solution is lower than that of its pure solvent, is called Diffusion Pressure Deficit (D.P.D.).
In case of D.P.D., the solution will try to wipe off this difference in D.P. by sucking more water (solvent) molecules and hence D.P.D. is the measure or index of sucking power and hence it is also called suction pressure (S.P.)
So D.P.D. = S.P.
(Term suction pressure was given by Renner).
Relationship Between D.P.D., (S.P.), Osmotic Pressure & Turgor Pressure (Wall Pressure)
D.P.D. (S.P.) = O.P. - T.P. (W.P.)
Initially (or in a flaccid cell), T.P. = 0
So, D.P.D. (S.P.) = O.P.
Therefore cell absorbs or sucks water with a force equal to O.P.
• In a fully turgid cell D.P.D. =.0
As, T.P. = O.P.
i.e., cell has no further capacity to absorb any water
Concept Of Water Potential, Solute Potential And Pressure Potential
Concept of water potential was given by Slatyer and Taylor.
It is the difference in the free energy (chemical potential) of water molecules in the solution and that of pure water at the same temperature and pressure.
It is indicated by the Greek letter Psi, the symbol for which is 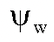 .
. 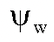 of pure water is zero.
of pure water is zero. 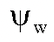 of a solution is always negative.
of a solution is always negative.
Solute Potential (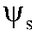 )
)
It is defined as the amount by which the water potential is reduced as a result of the presence of solute. Solute potential is also known as osmotic potential which is always expressed in negative values and is represented in bars. Osmotic pressure and osmotic potential are numerically equal but osmotic potential has a negative sign.
Pressure Potential ( )
)
The pressure potential operates in plant cells as wall pressure and turgor pressure and usually has a positive value.
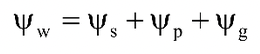
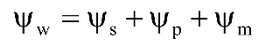
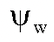 = Water potential
= Water potential
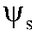 = Solute potential or osmotic potential
= Solute potential or osmotic potential
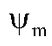 = Matric potential
= Matric potential
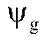 = Gravitational potential
= Gravitational potential
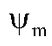 and
and 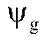 are negligible
are negligible
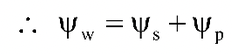
Where, 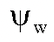 and
and 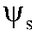 are negative values.
are negative values.
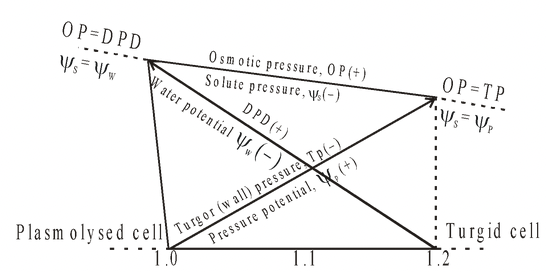
fig : Relation between OP (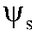 )1 TP (WP or =
)1 TP (WP or = ) and DPD (=
) and DPD (=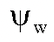 ) of a cell. Note that DPD (–
) of a cell. Note that DPD (–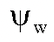 ) is zero when Tp (
) is zero when Tp ( ) becomes equivalent to OP (=
) becomes equivalent to OP (=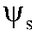 ) when TP (=
) when TP (= ) is nil as in plasmolysed cell.
) is nil as in plasmolysed cell.









