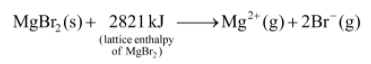IONIC OR ELECTROVALENT BOND
Chemical Bonding of Class 9
IONIC OR ELECTROVALENT BOND
The ionic bond is formed due to the electrostatic attraction between stable ions formed by the complete transfer of electrons from one atom to another. Let us consider the sodium atom who’s having one valance electron. As described above during the formation of a bond the atoms are first converted into ions, which generally have noble gas configurations. The sodium atom does so by losing its one outer most electron and thus it is converted into sodium cation .
Na → Na+ + electron − Ei (Ei = ionization energy)
(Sodium atom) (Sodium ion)
On the other hand chlorine atom having 7 electron in valance shell is short of one electron of the stable inert gas configuration and thus chlorine atom tends to gain an electron.
Cl + electron → Cl− + EA (EA = Electron Affinity)
(Chlorine atom) (Chloride ion)
The Na+ and Cl− having inert gas configurations are held together by the electrostatic attraction of their opposite charges.
Na+ + Cl−  [Na+] [Cl−] + Q cal ΔH = −Q cal
[Na+] [Cl−] + Q cal ΔH = −Q cal
[Sodium chloride]
Factors Influencing the formation of Ionic bond: The following factors facilitate the formation of an ionic bond between a metal and a non metal atom.
(i) Ionisation Energy: It is defined as the amount of energy required to remove the most loosely bound electron from an isolated gaseous atom of an element. The lesser the ionization energy, the greater is the ease of the formation of cation. Alkali metals and alkaline earth metals have low ionization energies and therefore form metal cations very easily.
M(g)  M+(g) + e−− Ei (Ei = Ionisation energy)
M+(g) + e−− Ei (Ei = Ionisation energy)
(ii) Electron Affinity: It is defined as the amount of energy released when an electron is added to an isolated gaseous atom of an element. The formation of an anion occurs with the addition of one or more electrons to an atom generally a non−metal atom. The greater the energy released during this process, the easier will be the formation of an anion, evidently, thus high electron affinity of a non−metal favours the formation of an anion.
X(g) + e− X−(g) + EA (EA = Electron Affinity)
X−(g) + EA (EA = Electron Affinity)
Factors affecting the magnitude of electron affinity
i) Atomic size – In general electron affinity value decreases with the increasing atomic radius because electrostatic force of attraction decreases between the electron being added and the atomic nucleus due to increase of distance between them.
Electron affinity α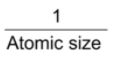
ii) Effective nuclear charge – Electron affinity value of the element increase as the effective nuclear charge on the atomic nucleus increases because electrostatic force of attraction between the electron being added and the nucleus increases. As the electrostatic force of attraction increases, amount of energy released is more.
Electron affinity α Effective nuclear charge (Zeff)
iii) Screening or Shielding effect – Electron affinity value of the elements decreases with the increasing shielding or screening effect. The shielding effect between the outer electrons and the nucleus increases as the number of electrons increases in the inner shells.
Electron affinity α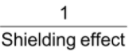
iv) Stability of half filled and completely filled orbitals – The stability of half filled and completely filled degenerate orbitals of a sub shell is comparatively more, so it is difficult to add electron in such orbitals and lesser energy is released on addition of electron hence the electron affinity value will decrease
(iii) Lattice Energy: It is defined as the amount of energy released when cations and anions are brought from infinity to their respective equilibrium sites in the crystal lattice to form one mole of the ionic compound in the solid state. Higher the magnitude of the lattice energy, the greater is the tendency of the formation of an ionic bond.
M+(g) + X−(g)  MX (s) + EL(EL = Lattice energy)
MX (s) + EL(EL = Lattice energy)
Properties of Ionic Compounds
- Ionic solids are well−defined crystalline solids. They are hard but brittle solids. They are composed of charged atoms or group of atoms as single ions of a non−metal. Separate units of ionic compounds do not exist. Therefore ionic compounds do not contain molecules. They have formula units and the formula only indicates the ratio of number of ions. Thus NaCl is not the molecule of sodium chloride but represents its formula unit. Hardness of ionic solids is due to the strong electrostatic forces of attraction between oppositely charged ions,
- Ionic compounds have high melting and boiling points. There exist strong forces of attraction between the ions in the crystal of an ionic compound. The ionic bond is very strong and hence a large amount of thermal energy (high temperature) is required to overcome these forces and break down the crystal lattice.
- Ionic compounds are generally soluble in water and other polar solvents (solvents with high di−electric constant which separate ions). They are practically insoluble in organic solvents such as benzene, carbon tetrachloride, carbon disulphide etc as there is no attraction between ions and the molecules of non−polar solvents. When an ionic compound dissolves in water, the ions get hydrated and the energy evolved is hydration energy.
- For dissolution of an ionic solid in water the necessary condition is
Hydration Energy > Lattice Energy
Ionic compounds such as Ca3(PO4)2, CaF2, BaSO4, CaCO3, etc. are insoluble in water due to their high lattice energies.
- Ionic Compounds do not conduct electricity in the solid state although they are composed of ions. It is because the ions are held by strong forces of Coulombic attraction hence can not act as carrier of electric current. In aqueous solution or molten state they become conductors of electricity because the attractive forces between the ions are cut off and the component ions become mobile. These free ions are capable of movement under the influence of electric field and thus act as carriers of electric current.
- The chemical properties of an ionic compound are the properties of its constituent ions. Thus all the chlorides respond to the reactions of the chloride ions. For example they produce white ppt. of AgCl in reaction with AgNO3 solution.
- In solution ionic compounds show ionic reactions which are quite fast and are instantaneously completed.
- Ionic compounds do not show stereoisomerism because ionic bond is non−directional.
Example-1: Calculate the lattice enthalpy of MgBr2 from the following data :
Mg(s)  Mg(g),
Mg(g), 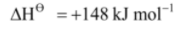
Mg(g) .png) Mg2+(g) + 2e–,
Mg2+(g) + 2e–, 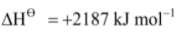
Br2(l)  Br2(g),
Br2(g), 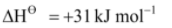
Br2(g)  2Br(g),
2Br(g), 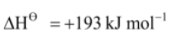
Br(g) + e– Br–(g),
Br–(g), 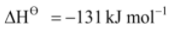
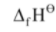 [MgBr2(S)] = – 524 kJ mol–1
[MgBr2(S)] = – 524 kJ mol–1
Solution: Born-Haber cycle for the calculation of lattice enthalpy of MgBr2 is shown below :
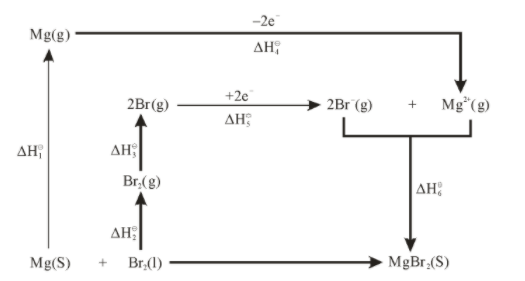

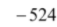
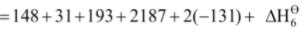
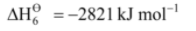
Hence, lattice enthalpy = – 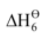 = + 2821 kJ mol–1
= + 2821 kJ mol–1
i..e,