
Nutrient Cycle
Ecosystem of Class 12
All living organisms get matter from the biosphere components i.e. lithosphere, hydrosphere and atmosphere. Essential elements or inorganic substances are provided by earth and are required by organisms for their body building and metabolism, they are known as biogeochemicals or biogenetic nutrients.
Some nutrients like carbon, nitrogen and phosphorus are required in large amounts and are called macronutrients, while some other nutrients like zinc, molybdenum, copper, boron etc. are required in very small amounts and are called micronutrients.
As the matter is neither created nor destroyed, as the materials must have been used again and again in the formation of organisms. We can say that matter is recycled. The exchange of materials between living and non-living components of biosphere is called biogeochemical cycle. These nutrients flow from non-living and back to the non-living in a circular fashion.
There are two types of biogeochemical cycles :
- Gaseous cycles
- Sedimentary cycles
Gaseous cycles : Generally perfect cycles and the nutrients self-adjust quickly due to presence of large reservoir.
Sedimentary cycles : Generally imperfect cycles as bulk of nutrient remains immobile on the earth’s crust.
COMMON BIOGEOCHEMICAL CYCLES
Biogeochemical cycles (Gr. bio = life; geo = earth; chemicos = chemical elements) are the cyclic pathways through which chemical elements move from environment to organism and back to the environment. Such movements of elements and inorganic compounds is essential for maintenance of life, so these are also called nutrient cycles.
Each bio-geochemical cycle is comprised of two types of pools :
- Reservoir pool : It is the large, slow-moving and generally non-biological.
- Exchange or cyclic pool : It is small, fast moving component which is exchanging rapidly between organisms and their immediate environment.
On the basis of movement of nutrients, bio-geochemical cycles are of 2 types :
- Perfect cycles : In these cycles e.g. gaseous cycle nutrients remain in circulation more or less uniformly.In these cycles nutrients self-adjust rather quickly because of large reservoir.
- Imperfect cycles : These cycles get more easily disrupted by local disturbances as the bulk of materials remains in the relatively inactive and immobile reservoir on the earth’s crust e.g. sedimentary cycles of phosphorus, sulphur, calcium, potassium, etc.
THREE ASPECTS OF A NUTRIENT CYCLE
Nutrient cycles can be conveniently considered under the following three aspects.
- Input of nutrients
- Output of nutrients
- Internal nutrient cycling
INPUT OF NUTRIENTS
In this, an ecosystem receives the nutrients from external sources and stores them for their reutilisation in the biological processes for the growth and development of living organisms.
Main sources of input of nutrients are :
- Wet deposition : In this, nutrients are received in dissovled state along with the rainfall.
- Dry deposition : In this, nutrients enter into an ecosystem in particulate state from dust fall. Symbiotic biological fixation of nitrogen in soil. Release of nutrients from their fixed state by weathering of rocks.
OUTPUT OF NUTRIENTS
In this, nutrients are moved out of an ecosystem e.g.Loss of nutrients like calcium, magnesium, etc. through run off water and soil erosion. Denitrification (conversion of nitrites and nitrates into ammonia and then into free nitrogen into the air) by certain bacteria. e.g. Pseudomonas.During harvesting of agricultural crops or transportation of logs from forests. An undisturbed ecosystem is generally in homeostasis because in these there is a physiological balance between the input of nutrients and output of nutrients. This keeps the nutrient cycle more or less balanced. But major disturbances like falling of trees, outbreak of fire or pathogenic insects, soil erosion, etc. may make them unbalanced and unstable.
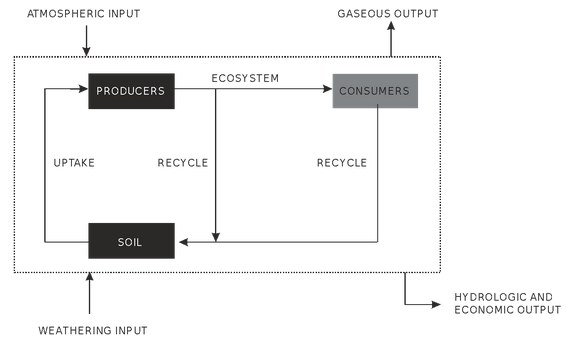
Fig. : A generalised model of nutrient cycling in an ecosystem
INTERNAL NUTRIENT CYCLING
It involves the following steps :
Regeneration of nutrients during decomposition of detritus by the decomposers like bacteria and fungi. These nutrients are stored in the soil for further utilization. Nutrients undergo both absorption and regeneration in the soil due to which a dynamic state of nutrients is maintained. Nutrient absorption involves uptake of nutrients from soil by the plants to incorporate them in their growth and development.
Sometimes, nutrients are recycled back into the soil during the process of litter fall from the plant sources, animal remains and faecal matter of the animals.
Above ground detritus and root detritus are decomposed to regenerate the nutrients to be reused by the plants. When the rate of uptake of nutrients is more than the amount of nutrients recycled (e.g. in a young growing forest), then a part of uptake nutrients is retained in the standing crop. This is called retention of nutrients. It increases the amount of nutrients in the ecosystem.
So,
Retention = Uptake – Recycle
Rates of nutrient uptake, recycle and retention are different in different ecosystems. These can be determined by a number of chemical methods. By comparing the changes in concentration of nutrients present and biomass with time, then the nutrient budget of the ecosystem can be determined.
NITROGEN CYCLE
Nitrogen is used as raw material for the synthesis of amino acids, proteins, enzymes, nitrogenous bases, hydrogen acceptors NAD, NADP, FAD), nucleic acids, ATP, photosynthetic pigments, cytochromes, phytochromes, growth hormones, alkaloids, etc., and thus is a very important raw material or nutrient for both plants and animals. Plants absorb nitrogen usually in the form of nitrates from the soil solution. It is reduced to ammonia and then converted into proteins and enzymes in the protoplasm.
Nitrogen is also used in the synthesis of photosynthetic pigments, chlorophylls, in the leaves of plants. Deficiency of nitrogen develops chlorosis in old leaves, early defoliation, stunted growth (due to deficiency of auxins), delaying in flowering and early death.
The nitrogen cycle can be conveniently discussed under heads they are :
- Nitrogen fixation
- Ammonification
- Nitrification
- Denitrification
NITROGEN FIXATION
Atmosphere is the greatest reservoir of nitrogen (approximate 78%). Atmospheric nitrogen is usually not used by the common plants. Only nitrogen fixing bacteria and blue green algae have the ability of fixing atmospheric nitrogen to convert it into organic nitrogen in their cell protoplasm.
Nitrogen fixing bacteria are :
- Clostridium : Anaerobic, free-living and soil borne
- Azatobactor : Aerobic, free-living, saprophytic and soil borne
- Rhizobium : Symbiotic, saprophytic and soil borne
- Klebsiella : Symbiotic, saprophytic and air borne
- Chlorobium : Anaerobic, autotrophic and free-living
- Rhodospirillum : Anaerobic, autotrophic and free-living
- Aerobactor : Anaerobic, heterotrophic and soil borne

Fig. : Nitrogen Cycle
Nitrogen fixing blue-green algae are :
- Nostoc
- Anabaena
- Aulosira
- Gloeotrichia
- Calothrix
- Rivularia etc., (all heterocystous, filamentous forms).
Nitrogen fixation is a complex biological process in which the atmospheric nitrogen is first converted to ammonia and then to amino acids which form the building blocks of proteins. Proteins are incorporated in protoplasm in various forms enzymes, cytochromes, chromatin materials, ribosomes and membrane systems. After the death of cell, proteins are subjected to microbial decomposition in which ammonia is released which is then oxidized to form nitrites and nitrates with the help of nitrifying bacteria.
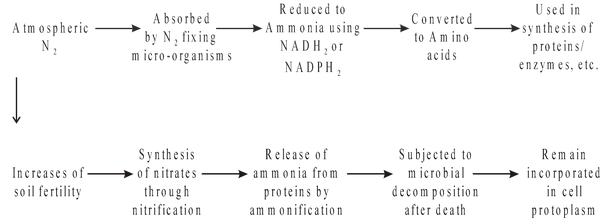
Mechanism of Biological N2 Fixation
AMMONIFICATION
Proteinaceous organic remains contained in the dead organic matter of plants and animals in soil are decomposed by many fungi Clostridia, Actinomycetes and Proteus. Proteolysis of proteins results in formation of aminoacids and finally ammonia is produced by oxidative deamination. If proteins are decomposed anaerobically (putrefaction), they produce amides and then ammonia is produced by their oxidation. Ammonia as such is poisonous and thus its accumulation in soil is harmful. It is oxidised to nitrate through nitrification.
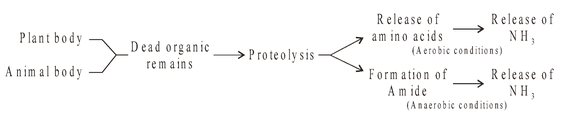
NITRIFICATION
Oxidation of ammonia to form nitrite and then to nitrate is called nitrification which is carried out by two specific groups of chemosynthetic bacteria, as follows :
2NH3 + 3O2 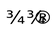 2HNO2 + 2H2O +70 kcal energy
2HNO2 + 2H2O +70 kcal energy
2HNO2 + O2 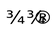 2HNO3 + 20 kcal energy
2HNO3 + 20 kcal energy
- By Nitrosomonas, Nitrosococcus, Nitrosospira and Nitrosocystis (commonly called Nitrosomonas group) ammonia is converted to nitrites
- By Nitrobacter group of bacteria. Streptomyces, Nocardia, Penicillium, Aspergillus, Cephalosporium, like heterotrophic forms have also shown nitrifying ability. They convert nitrite to nitrates.
DENITRIFICATION
The biological process by which ammonia compounds, nitrates and nitrites are reduced to molecular nitrogens is called denitrification. Thiobacillus denitrificans and Pseudomonas denitrificans are capable of converting all bound forms of nitrogen, organic or inorganic, to free gaseous nitrogen for recycling. They, thus, reduce soil fertility but play an important role in nitrogen cycle. Nitrogen loss from soil by denitrification occurs during seasonal flooding of the land or during excessive irrigation.

Green plants use filtrate of the soil. It is assimilated in cell proteins, pigments, hormones and membranous system. It forms the important component of plant biomass. Herbivores obtain nitrogen from plant biomass and during the digestion, this protein is hydrolysed to form free amino acids. Specific proteins in the body of herbivores are synthesised by rearranging these free amino acids. From the herbivores, proteins come to the body of carnivores through food chain. Top consumers obtains protein from the flesh of herbivores as well as carnivores. The different trophic levels of the ecosystem store nitrogen in their biomass as their body proteins.Some of the proteins of the animal organisms are hydrolysed to form ammonia is then converted to urea and excreted through urine.
The plant and animal biomass, which remains unutilized, is subjected to microbial decomposition after their death aerobically or anaerobically to release ammonia in soil where nitrifying bacteria convert ammonia into nitrite and finally into nitrate which becomes the raw material for plants and is thus reused. When excess of nitrate ammonia are accumulated in soil, denitrifying bacteria decompose them to form nitrogen gas for recycling. Biological nitrogen fixation is an additional pathway for increasing soil fertility.
Human beings sometimes interfere with nature’s nitrogen cycle either by burning the dead organic remains lying on the floor of soil or by adding nitrogenous manures exogenously to increase soil fertility and yield. Under slow rain conditions, some of the nitrates of soil are gradually leached away. Uncontrolled and accelerated water erosion from agriculture fields may often cause tremendous loss of nitrogen from soil. Excess of nitrates in drinking waters often causes ‘Blue Baby Syndrome or methamoglobinemia’ disease in child.
