ELECTRIC CHARGE
Electricity of Class 9
Electric charge, basic property of matter carried by some elementary particles. Electric charge, which can be positive or negative, occurs in discrete natural units and is neither created nor destroyed.
When we rub our shoes across a carpet and reach for a metal doorknob, we can be zapped by a spark of electricity. The answers to this lies in the branch of Physics called Electrostatics. The word electricity comes from the Greek word electron, which means "amber." Amber is petrified tree resin, and it was well known to the ancients that if we rub an amber rod with a piece of cloth, the amber attracts small pieces of dry leaves or paper. A piece of hard rubber, a glass rod or a plastic comb rubbed with cloth also display this "amber effect" or static electricity or frictional electricity as we call it today.
Experiments show that there are exactly two kinds of electric charges :
- Negative charge
- Positive charge
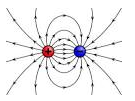
These also show that unlike charges attract each other while like charges repel each other. The S.I. unit of electric charge is coulomb. It is denoted by symbol C.
Electrons
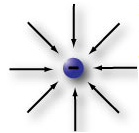
Electrons are the smallest and lightest of the particles in an atom. Electrons are in constant motion as they circle around the nucleus of that atom. Electrons are said to have a negative charge, which means that they seem to be surrounded by a kind of invisible force field. This is called an electrostatic field.
Protons
Protons are much larger and heavier than electrons. Protons have a positive electrical charge. This positively charged electrostatic field is exactly the same strength as the electrostatic field in an electron, but it is opposite in polarity. Notice the negative electron (pictured at the top left) and the positive proton (pictured at the right) have the same number of force field linesin each of the diagrams. In other words, the proton is exactly as positive as the electron is negative.
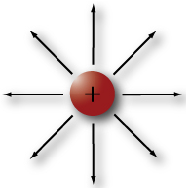
Like charges repel, unlike charges attract
Two electrons will tend to repel each other because both have a negative electrical charge. Two protons will also tend to repel each other because they both have a positive charge. On the other hand, electrons and protons will be attracted to each other because of their unlike charges.
Since the electron is much smaller and lighter than a proton, when they are attracted to each other due to their unlike charges, the electron usually does most of the moving. This is because the protons have more mass and are harder to get moving. Although electrons are very small, their negative electrical charges are still quite strong. Remember, the negative charge of an electron is the same as the positive electrical charge of the much larger in size proton. This way the atom stays electrically balanced.
Another important fact about the electrical charges of protons and electrons is that the farther away they are from each other, the less force their electric fields have on each other. Similarly, the closer they are to each other, the more force they will experience from each other due to this invisible force field called an electric field.
- ELECTRICITY
- CONDUCTORS AND INSULATORS
- ELECTRIC CIRCUIT AND ITS COMPONENTS
- EARTHING
- ELECTRIC CHARGE
- PROPERTIES OF ELECTRIC CHARGE
- ELECTRIC CURRENT
- ELECTRIC FIELD AND ELECTRIC POTENTIAL
- OHM’s LAW
- COMBINATION OF RESISTORS
- Solved questions
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4 (True and False)
- Exercise 5 (Subjective)









