Green House Effect And Global Warming
Environmental of Class 11
Green House Effect And Global Warming
The term green house effect had its origin from the practice of encasing vexampleetation in glass chambers to protect them from frost particularly in cold countries. It was observed that there was a continued rise in temperature in such chambers even when the outside temperature remains low. Thus enabled the warming up of vexampleetation inside the chamber, resulting in good plant growth.
Green house effect around the earth
Atmosphere around the earth acts like a glass of the green house chamber. The gases present in the atmosphere which cause green house effect are referred to as green house gases. The various green house gases are:
CO2 ,HO2 vapours, chlorofluoro carbons and oxides of nitrogen.
The green house gases in the atmosphere form a thick cover around the earth. The earth receives a large amount of energy from the sun. The IR radiations coming from sun are not absorbed by atmospheric gases. The earth absorbs these IR radiations of short wavelength. As a result of this the temperature of earth stands rising. Eventually, earth starts emitting infrared radiations of longer wavelengths. The partially radiated infrared radiations from the earth are absorbed by CO2. This results in excessive heating of earths atmosphere.
The heating of atmosphere due to absorption of infrared radiations by CO2 and other gases is called green house effect.
Advantages of green house effect
The presence of CO2 and other gases in the atmosphere produce green house effect, which in turn keeps the atmosphere warm. The warm atmosphere is very essential for survival of life on earth in the following ways:
(i) It is necessary for evaporation of water, formation of clouds, rainfall etc.
(ii) The warm atmosphere helps in rapid growth of plants, trees etc.
(iii) Warm atmosphere helps is biodexampleradation of dead plants and animals.
Harmful effects of green house effect
The disastrous consequences of green house effect are following:
(i) High temperature of atmosphere may melt polar ice caps which is likely to raise the level of sea thereby sinking most of the coastal areas and causing large scale destruction.
(ii) The high temperature may reduce crop product.
(iii) The high temperature will reduce work efficiency of human being.
(iv) Tropical rains and hurricane will become more frequent and also more stronger causing more devastation.
(v) The change in ocean temperature will adversely affect the warm life.
Water Pollution
The quality of drinking water is very important for human welfare. The pollution of water by sewage has been linked to the spread of diseases such as cholera & typhoid fever. The given table lists the major water pollutants and their sources.
|
Pollutant |
Source |
|
Microorganism |
Domestic sewage |
|
Organic wastes |
Domestic sewage, animal waste, decaying animals, plants and discharge from food processing factories |
|
Plant nutrients |
Chemical fertilizers |
|
Toxic heavy metals |
Industries and chemical factories |
|
Sediments |
Erosion of soil by agriculture and strip mining |
|
Pesticides |
Chemical used for killing insects, fungi & weeds |
|
Radioactive substances |
Mining of Uranium containing minerals |
|
Heat |
Water used by industrial plants which is discharged as hot water |
In addition, industrial waster also contaminate water.
(i) Heavy Metals
Metals such as Cd, Pb & Hg may be present in industrial or mining waste. These metals can prove poisonous to humans – cadmium and mercury can cause kidney damage and lead poisoning can cause damage to the kidneys, liver, brain and central nervous system.
(ii) Detergents & fertilizers
They may contain phosphates as additives. The addition of phosphorus to water in the form of the phosphate anion PO3-4 encourages the formation of algae which reduces the dissolved oxygen concentration of water. This process is known as eutrophication.
(iii) Acid polluted water (pH < 3)
This is deadly to most forms of aquatic life. Water down stream from a mine may be contaminated by acid mine drainage, the result of microbial oxidation of discarded waste material at the mine site. Industrial wastes and acid rain may also contribute to the acidity of natural waters.
(iv) Poly chlorinated biphenyls (PCBs)
These chemicals are relatively recent additions to the list of contaminants of water. Having high stabilities, PCB’s find many applications PCB’s are resistant to oxidation and their release into the environment causes skin disorders in humans. They are reported to be carcinogenic.
Importance of dissolved oxygen in water
The concentration of dissolved oxygen in water is highly important for the support of aquatic life. The lower is concentration of dissolved oxygen, the more polluted is the water sample. Oxygen gets into water through two sources:
(i) Dissolution of oxygen at the surface of water from the atmosphere.
(ii) From photosynthesis of aquatic plants.
Deoxygenation of water
The dissolved oxygen in water is consumed rapidly by microorganisms to oxidise organic matter of sewage.

Oxygen in water may be consumed by the bio – oxidation of the nitrogenous material and by the chemical or bio chemical oxidation of chemical reducing agents such as Fe2+ ions or SO2-3 ions.
Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD)
The polluted water may contain large amounts of inorganic and organic compounds. Some of these can be oxidised by dissolved oxygen in the presence of microorganisms.
BOD, is a measure of the dissolved oxygen that would be needed by the microorganisms to oxidise these compounds. BOD, therefore is a measure of the total contamination caused by compounds which can be oxidised in the presence of microorganisms. The BOD is taken as a realistic measure of water quality – clean water would have a BOD value of less than 5 ppm whereas highly polluted river water could have a BOD value of 17 ppm or more.
BOD Determination
In order to find out BOD, the water sample is first saturated with oxygen. It is then incubated at constant temperature. This allows time for microorganisms in water sample to oxidise pollutants. The remaining amount of dissolved oxygen is determined and BOD is obtained by subtraction BOD measurements takes a few days, so another parameter called the chemical oxygen demand (COD) is sometimes measured.
COD determination
In COD determination the water sample is treated with a known quantity of an oxidising agent, usually K2Cr2O7 in acidic medium. This reagent oxidises most of the polluting substances, including those which are resistant to microbial oxidation. The remaining K2Cr2O7 is determined by back titration with a suitable reducing agent. From the concentration of K2Cr2O7 consumed, the amount of oxygen used in oxidation may be calculated using the following chemical equation:
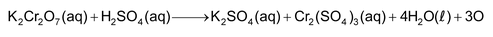
The results are expressed in terms of amounts of oxygen in ppm, that would be required to oxidise the contaminants. This is called COD.
Control of water pollution
Some of the steps which are helpful for the control of water pollution are being described as follows:
(i) Waste water treatment techniques can be applied before the polluted water enters a river, lake or pool.
(ii) Septic tanks should be used for each house.
(iii) Rivers, lakes etc. should not be used for bathing and washing purposes.
(iv) Too much use of pesticides which are not biodexampleradable should be avoided. These are highly toxic substances.
(v) Efforts should be made to increase the use of low grade or polluted water.
Land Pollution
Most of the land pollution is caused by pesticides and other chemicals which are added to the soil to grow better crops. Often a pesticides poisons many more organism than those intended. Some of these poisons passes through food chains and eventually reach harmful proportions. Solid wastes is another cause of land pollution.
Pesticides
Pesticides are substances that are used to kill or block the reproductive processes of unwanted organisms. Synthetic pesticides are of concern to us, because of the possible effect upon human health through eating of food or drinking water, contaminated with these chemicals. Most pesticides can put into one of three catexampleories :
(i) Insecticides
Control of insects by insecticides helps to curb disease and protect crops. Organo chlorines are a group of compounds which have been developed and used as insecticides. The best known organochlorine compound is DDT (dichlorodiphenyl trichloro ethane) organo chlorines are stable in the environment, toxic to insects in small amounts, but much less go to humans, and because they are organic compounds not very soluble in water. The advantage of these insecticides is that, bring persistent, they show their biological activity for long periods of time. On the nexampleative side, these insecticides by accumulating in the environment also affect many non – target organisms, not just the target pests.
(ii) Fungicides
Fungicides are used to check the growth of fungi. Fungi, are plants without chlorophyll, they cannot use solar energy for preparing their food. They live as saprophytes on decaying organic matter or as parasites at the expense of living organisms. Hence they are considered to be a threat to human interests. Fungicides are important because they check the growth of fungi. Organic compounds of Mercury have been used as fungicides. These compounds break down in soil and thus have many disastrous consequences.
Control of soil pollution
The following steps have been suggested to control the soil pollution:
(i) The use of chemical fertilizers can be reduced by applying bio – fertilizers and manures.
(ii) Recycling and recovery of materials appears to be a reasonable solution for reducing soil pollutions. Materials such as paper, gas, and some kinds of plastics can be recycled.
(iii) Control of land loss can be attempted through restoring forest and grass cover to check soil erosion and floods.
(iv) Proper methods should be adopted for the disposal of solid wastes.
Green Chemistry: A new route to protection of environment
The term green chemistry may be defined as the program of developing new chemical products and chemical processes or making improvement in the already existing compounds and processes so as to make them less harmful to human health and environment.
For attaining success in objectives of green chemistry, following particles are to be used
(i) The use of starting materials, reagents and solvents which are less hazardous to man and his environment.
(ii) More efficient use of raw materials.
(iii) Utilisation of chemical reactions which completely incorporate the starting materials into the final products.
(iv) Search for new alternatives which are environmentally friendly.
Collective efforts in the field of green chemistry have made tremendous impacts on several industrial sectors in recent years. Some of the achievements in this field are as follows:
(i) Development of dense phase, carbon dioxide: Dense phase CO2 has been recently developed chemical product with amazing characteristics. It has ability to clean everything. It can be used as recyclable solvent and finds number of applications in food industry.
(ii) Development of fuel cells for cellular phones which can last for the full life time of the phone.
(iii) Development of process involving use of CO2 as a blowing agent for manufacture of poly styrene foam. This technology eliminates the use of chloro fluoro carbon as blowing agent.
(iv) Development of safer marine antifouling compound sea-nine that dexamplerades more rapidly than organotins which persists in marine environment and cause pollution Prob.s.
Thus Green Chemistry programs recognise and promote fundamental break through in chemistry that accomplish prevention in a cost effective manners.









