SSC Worksheet for chapter-3 Acids, Bases and Salts class 10
Worksheet For class 10
This page is prepared by the Academic team of Physics Wallah which consists of SSC Board Worksheet for Class 10 Chemistry. Students of Class 10 Chemistry can get a free Worksheet for Class 10 Chemistry in PDF format prepared as per the newest syllabus and examination pattern in your schools.
Before solving the Worksheet for class 10 one must read the textbook of your school board exam and try to read the notes given in Physics Wallah class 10 notes sections. Students can also download free pdf of Class 10 Chemistry Notes prepared by teachers and solve important problems provided here with solutions on daily basis to get more scores in school exams and tests.
Physics Wallah worksheets for class 10 SSC can help students practice with tests and quickly revise lessons learned in the classroom with essential resources such as video lessons, short notes, and sample papers.
Class 10 Board is one of the most important year for every student and need a good planning and right study material to score good marks in your final exam. If any students need to take the online test to check their concepts or undertstanding then they can visit Chemistry Quiz for Class 10.
Objective Type Questions
Q1. A solution reacts with crushed egg-shells to give a gas that turns lime water milky, the solution contains
- NaCl
- HCl
- LiCl
- KCl
Q2. Which one of the following types of medicines is used for treating indigestion ?
- Antibiotic
- Antacid
- Analgesic
- Antiseptic
Q3. What happens when a solution of an acid is mixed with a solution of a base in a test tube ?
- The temperature of the solution increases
- The temperature of the solution decreases
- The temperature of the solution remains same
- Salt formation takes place
Q4. An aqueous solution turns red litmus solution blue excess addition of which of the following solution would reverse the change ?
- Baking powder
- Ammonium hydroxide solution
- Lime
- Hydrochloride acid
Q5. Which of the following does not contain water of crystallization?
- Blue vitriol
- Baking soda
- Washing soda
- Crypsum
Q6. Sodium carbonate is a basic salt because it is a salt of
- Strong acid and strong back
- Weak acid and weak base
- Weak acid and strong base
- Strong acid and weak base
Q7. Calcium phosphate is present in tooth enamel, its nature is
- Basic
- Acidic
- Neutral
- Amphoteric
Q8. Which of the following substances will not give carbon dioxide on treatment with dilute acid ?
- Marble
- Lime
- Limestone
- Baking soda
Q9. Which one of the following can be used as an acid base indicator by a visually impased student ?
- Phenolphthalein
- Vanilla essence
- Litmus
- Turmeric
Q10. Which of the following phenomena occurs when a small amount of acid id added to water ?
- Ionisation
- Salt formation
- Neutralisation
- Dilution
Subjective Type Questions
Q11. What are Olfactory indicators ? Explain with examples ?
Q12. While diluting an acid, why is it recommended that the acid should be added to water and not water to acid ?
Q13. Why does diluted water not conduct electricity, whereas rain water does ?
Q14. Why milkman adds a very small amount of baking soda to fresh milk explain ?
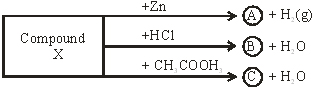
Q15. Identify the composed X on the basis of the reactions given below also write the name and chemical formula of A, B, C
Answer Key : Acids, Bases and Salts
|
1. (b) |
2. (b) |
3. (a, d) |
4. (d) |
5. (b) |
6. (c) |
7. (c) |
8. (b) |
9. (b) |
10. (a, d) |
Solutions
Ans 11. An Olfactory indicator is a substance whose smell varies depending on whether it is mixed into an acidic or basic solution, it can be used in laboratory to test whether a solution is a base or an acid. Onion and vanilla extract are example.
Ans 12. Dilution of a concentrated acid is a highly exothermic reaction a lot of heat is generated. Care must be taken while mixing concentrate acid with water, the acid must always be added slowly to water with constant stirring. If water is added to the concentrated acid, the heat generated may cause the mixture to splash out and cause burns the glass container may also break due to excessive local heating.
Ans 13. Distilled water is free from dissolved ions and hence, can’t conduct electricity. Rain water on the other hand dissolves atmospheric gases like CO2, SO2, NO2 etc to form acid like H2CO3, H2SO4 and HNO3 the amount of these acids is very low but since they can easily dissociate into ions they enable rain water to conduct electricity.
Ans 14. Milk sows easily at lower pH and gets curdled and spoiled so to prevent the spoilage of milk the milk man adds a very small amount of baking soda (alkaline) presence of baking soda makes milk alkaline for the conversion lactose to lactic acid by fermentation, which results in curd formation, an acidic pH is needed so this milk will take a longer time to set as curd.
Ans 15. X→NaOH (Sodium hydroxide)
Y→ NO2ZnO2 (Sodium zincate)
B→NaCl (Sodium chloride)
C→CH3COONO (Sodium acetate)
Related Link
- Class 10 Physics Notes
- Class 10 Chemistry Notes
- Class 10 Biology Notes
- Class 10 Maths Notes
- Chapter Wise Quiz Class 10 Physics
- Chapter Wise Quiz Class 10 Chemistry
- Chapter Wise Quiz Class 10 Biology
- Chapter Wise Quiz Class 10 Maths
- Previous year Board Papers
- Sample Papers Maths Class 10
- Sample Papers Science Class 10
- NCERT Maths Solutions Class-10









