SSC Worksheet for chapter-4 Metals and Non-Metals class 10
Worksheet For class 10
This page is prepared by the Academic team of Physics Wallah which consists of SSC Board Worksheet for Class 10 Chemistry. Students of Class 10 Chemistry can get a free Worksheet for Class 10 Chemistry in PDF format prepared as per the newest syllabus and examination pattern in your schools.
Before solving the Worksheet for class 10 one must read the textbook of your school board exam and try to read the notes given in Physics Wallah class 10 notes sections. Students can also download free pdf of Class 10 Chemistry Notes prepared by teachers and solve important problems provided here with solutions on daily basis to get more scores in school exams and tests.
Physics Wallah worksheets for class 10 SSC can help students practice with tests and quickly revise lessons learned in the classroom with essential resources such as video lessons, short notes, and sample papers.
Class 10 Board is one of the most important year for every student and need a good planning and right study material to score good marks in your final exam. If any students need to take the online test to check their concepts or undertstanding then they can visit Chemistry Quiz for Class 10.
Objective Type Questions
Q1. Which one of the following pairs will give displacement reactions?
- NaCl solution and copper metal
- MgCl2 solution and aluminium metal
- FeSO4 solution and silver metal
- Ag NO3 solution and copper metal
Q2. Which one the following metals do not react with cold as well as hot water?
- Na
- Ca
- Mg
- Fe
Q3. Which one of following oxide(s) of iron would be obtained on prolonged reaction of iron with steam?
- Feo
- Fe2O3
- Fe3O4
- Fe2O3& Fe3O4
Q4. What happens when calcium is treated with water?
- It does not react with water
- It reacts violently with water
- It reacts less violently with water
- Bubbles of hydrogen gas formed stick to the surface of calcium.
Q5. Composition of aqua-regia is
- Dil HCl: conc. HNO3(3:1)
- Conc. HCl: dil HNO3(3:1)
- Conc. HCl: conc. HNO3(3:1)
- Dil HCl : dil HNO3
Q6. Which of the following non-metal is a liquid?
- Carbon
- Brominc
- Phosphorous
- Sulphur
Q7. Which of the following metal form an amphoteric oxide?
- Na
- Ca
- Al
- Cu
Q8. Which of the following non-metal is lustrous?
- Sulphur
- Oxygen
- Nitrogen
- Iodine
Q9. In stainless steel, iron is mixed with
- Ni and Cr
- Cu and Cr
- Ni and Cu
- Cu and Au
Q10. Which of the following are not ionic compounds?
- KCl
- HCl
- CCl4
- NaCl
Subjective Type Questions
Q11. A metal A, which is uses in termite process, when heated with oxygen gives an oxide B, Which is amphoteric in nature, identify A and B. write down reactions of oxide B with the and NaOH.
Q12. Give the reaction involved during extraction of zinc from its ore by
- Roasting of zinc ore
- Calcinations of zinc ore.
Q13. What are constituents of solder alloy? Which property of solder makes it suitable for welding electrical wires?
Q14. Explain the following
- Reactivity of Al decrease of dipped in HNO3?
- Difference between ores and minerals
- Tarnished copper utensils are cleansed with time juice or tamarind
Q15. Give the formulae of the stable binary compounds that would be formed by the combination of following pairs of elements
- Mg and N2
- Li and O2
- Al and Cl2
- K and O2
Answer Key : Metal and Non-metals
| 1. (d) | 2. (d) | 3. (c) | 4. (c, d) | 5.. (c) | 6. (b) | 7. (c) | 8. (d) | 9. (a) | 10. (b, c) |
Solutions :
Ans 11. A is aluminium (Al) and
B is Al2O3 (aluminium oxide)
- Al2O3 + 6HCl→2 AlCl3 + H2O
- Al2O3 + 2 NaOH→2 NaAlO2 + H2O
Ans 12. (a) Roasting 2ZnS + 3O2 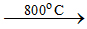 2ZnO + 2SO2
2ZnO + 2SO2
Zno + C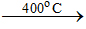 Zn + CO(g)
Zn + CO(g)
Calcination ZnCO3 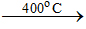 Zno + CO2
Zno + CO2
Zno + C .jpg) Zn + CO
Zn + CO
Ans 13. Solder is an alloy of Pb (lead) and Sn (tin), its lower melting point makes it suitable for welding electrical wires.
Ans 14. Because HNO3 renders aluminium passive due to the formation of an oxide film on its surface.
- An ore is a mineral from which metal can be extracted economically. While mineral is native form in which metal exist.
All ore are mineral, but all minerals are not necessarily ore.
e.g. Aluminium is extracted from the ore bauxite.
- When copper utensils are exposed to moist air, they get tarnished or corroded due to the formation of green copper carbonate. When these tarnished vessels are rubbed with lime juice or tamarind the weak acids present in then dissolve the green copper carbonate and original shine returns.
Ans. 15. Mg3N2
- H2O
- AlCl3
- K2O
Related Link
- Class 10 Physics Notes
- Class 10 Chemistry Notes
- Class 10 Biology Notes
- Class 10 Maths Notes
- Chapter Wise Quiz Class 10 Physics
- Chapter Wise Quiz Class 10 Chemistry
- Chapter Wise Quiz Class 10 Biology
- Chapter Wise Quiz Class 10 Maths
- Previous year Board Papers
- Sample Papers Maths Class 10
- Sample Papers Science Class 10
- NCERT Maths Solutions Class-10









