Enzyme Inhibition
Enzymes of Class 11
Enzyme Inhibition
A variety of small molecules exists which can reduce the rate of an enzyme controlled reaction. They are called enzyme inhibitors.
Inhibition is a normal part of the regulation of enzyme activity within cells. Many drugs and poisons also act as enzyme inhibitors. Inhibition may be competitive or non-competitive. Non-competitive inhibition
may be reversible or non-reversible.
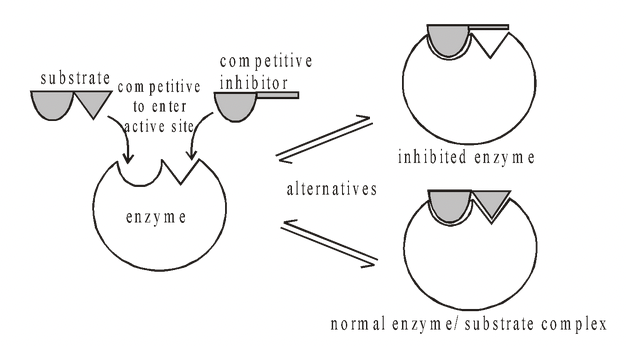
Competitive Inhibition
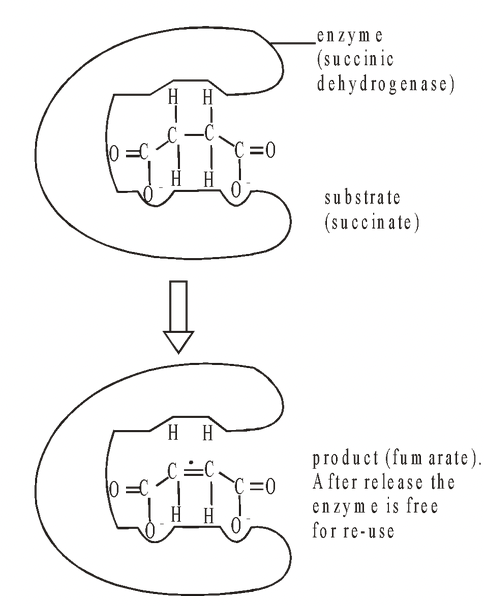
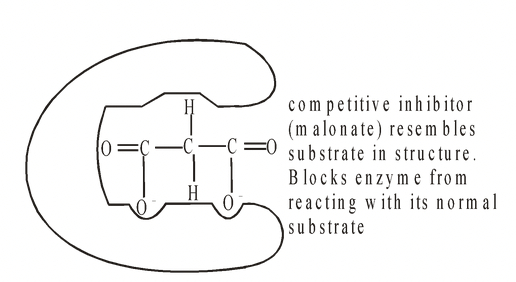
This occurs when a compound has a structure which is sufficiently similar to that of the normal substrate to be able to fit into the active site. Normally it does not take part in a reaction but while it remains there
it prevents the true substrate from entering the active site. The genuine substrate and the inhibitor therefore compete for a position in the active site, and this form of inhibition is called competitive inhibition. A
characteristic feature of competitive inhibition is that if the substrate concentration is increased, the rate of reaction increases.
The knowledge of competitive inhibition helps to understand the effect of a group of antibiotics known as sulphonamides.They are similar in structure to PAB (para-aminobenzoate), a substance essential for the growth of many pathogenic (disease-causing) bacteria. The bacteria require PAB for the production of folic acid, an important enzyme cofactor. Sulphonamides act by inhibiting an enzyme needed for the synthesis of folic acid from PAB.
Animal cells are insensitive to sulphonamides even though they require folic acid for some reactions. This is because they use pre-formed folic acid and do not possess the necessary metabolic pathway for making it.
Non-competitive Reversible Inhibition
Inhibitor has no structural similarity to the substrate and combines with the enzyme at a point other than its active site. It does not affect the ability of the substrate to bind with the enzyme, but it makes it impossible for catalysis to take place. The rate of reaction decreases with increasing inhibitor concentration. When inhibitor saturation is reached, the rate of the reaction will be almost nil. It is a characteristic of this type of inhibition that an increase in substrate concentration does not affect the rate of reaction, unlike with competitive inhibition.
Non-competitive Irreversible Inhibition
Some chemicals cause irreversible inhibition of enzymes. e.g. Very small concentrations of chemical reagents such as the heavy metal ions mercury (Hg2+), silver (Ag+) and arsenic (As+), or certain iodine-
containing compounds completely inhibit some enzymes. They combine permanently with sulphydryl (–SH) groups. These may be in the active site or elsewhere. Either way, the change in structure of the
enzyme makes it ineffective as a catalyst. The change may cause the protein of the enzyme molecule to precipitate.
Fig. Irreversible inhibition of enzyme by iodoacetic acid. The iodine reacts with sulphydryl groups
The nerve gas DFP (diisopropylfluorophospate) designed for use in warfare. It combines with the amino acid serine at the active site of the enzyme acetylcholinesterase. This enzyme deactivates the
neurotransmitter (needed to continue the passage of nerve impulses from one nerve cell to another across a synaptic gap) substance acetylcholine. When the impulse has been transmitted, acetylcholinesterase
functions to deactivate acetylcholine almost immediately by breaking it down. If acetylcholinesterase is inhibited, acetylcholine accumulates and nerve impulses cannot be stopped, causing prolonged muscle
contraction. Paralysis occurs and death may result since the respiratory muscles are among those affected. Some insecticides currently in use, including those known as organophosphates (such as parathion), have
a similar effect on insects, and can also cause harm to the nervous and muscular systems of humans who are overexposed to them.
Allosteric Enzymes
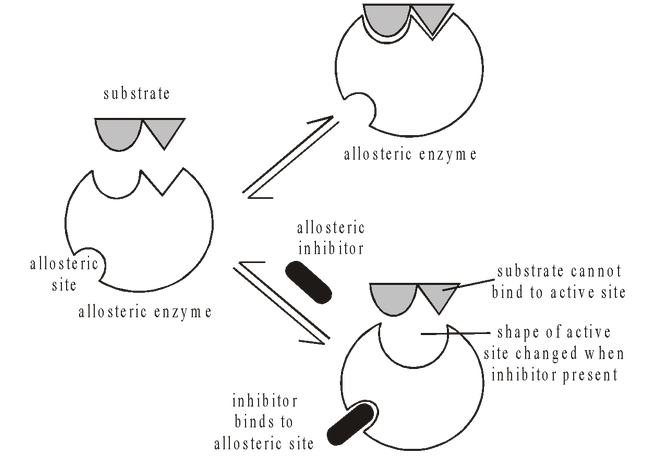
One of the commonest ways of regulating metabolic pathways in cells is by means of allosteric enzymes. These are enzymes which are ‘designed’ to change shape (allo, different; steric, shape). They are regulated by compounds which act as non-competitive inhibitors. These compounds bind to the enzyme at specific sites well away from the active site. They modify enzyme activity by causing a reversible change in the structure of the enzyme’s active site, which affects the ability of the substrate to bind to the enzyme (unlike non-competitive reversible inhibition). Compounds of this nature are called allosteric inhibitors.
An example of this is provided by one of the reactions of glycolysis (cell respiration). The purpose of cell respiration is to produce ATP. When ATP is at a high concentration, it inhibits one of the enzymes of glycolysis allosterically. However, when cell metabolism increases and more ATP is used up, the overall concentration of ATP decreases and the pathway once again comes into operation because the inhibitor, ATP, has been removed. This is also an example of end-product inhibition.
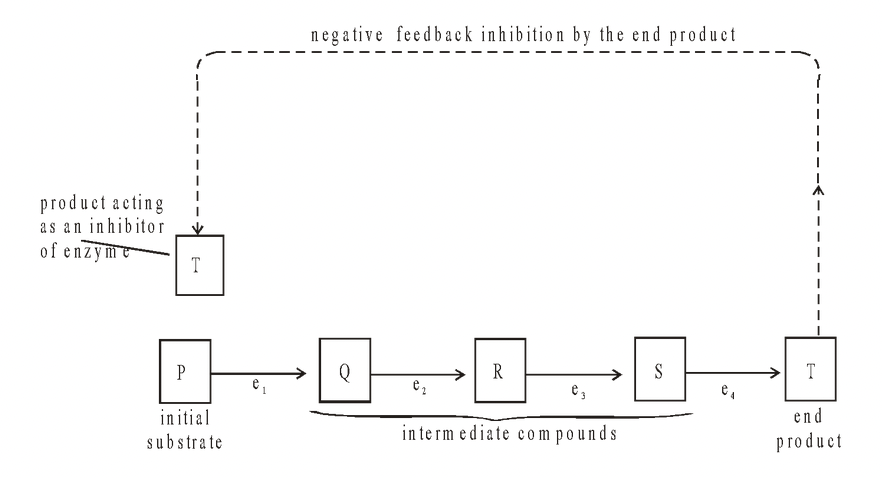
End-product Inhibition (negative feedback inhibition)
When the end product of a metabolic pathway begins to accumulate, it may act as an allosteric inhibitor of the enzyme controlling the first step of the pathway. Thus the product starts to switch off its own production as it builds up. The process is self-regulatory. As the product is used up, its production is switched back on again. This is called end-product inhibition and is an example of a negative feedback mechanism.