
Factors affecting the Rate of Enzyme
Enzymes of Class 11
Factors affecting the Rate of Enzyme
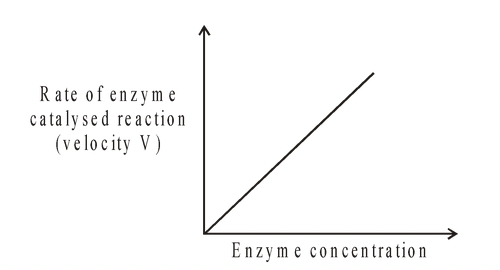
1. Enzyme concentration
Provided that the substrate concentration is maintained at a high level, and other conditions such as pH and temperature are kept constant, the rate of reaction is proportional to the enzyme
concentration. Normally reactions are catalysed by enzyme concentrations which are much lower than substrate concentrations. Thus as the enzyme concentration is increased, so will the rate of the enzyme reaction.
2. Substrate Concentration
For a given enzyme concentration, the rate of an enzyme catalysed reaction increases with increasing substrate concentration. The theoretical maximum rate (Vmax) is never quite obtained,
but there comes a point when any further increase in substrate concentration produces no significant change in reaction rate. This is because at high substrate concentrations the active sites
of the enzyme molecules at any given moment are virtually saturated with substrate. Thus any extra substrate has to wait until the enzyme/substrate complex has released the products
before it may itself enter the active site of the enzyme.
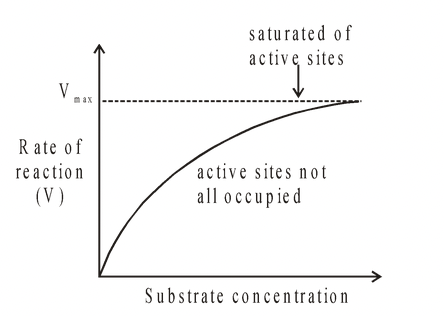
Fig. Effect of substrate concentration on the rate of an enzyme controlled reaction
Michaelis Menton constant (Km)
It is a mathematical derivation or constant which indicates the substrate concentration at which the chemical reaction catalysed by an enzyme attains half its maximum velocity.
Km constant generally lies between 10–1 to 10–6 M.
Km indicates affinity of the enzyme for its substrate. A high Km indicates low affinity while a low Km shows strong affinity. If an enzyme acts on more than one substrate it shows different Km values for them.
Thus enzyme protease acts on large number of proteins. Its Km value will differ from protein to protein.
Allosteric enzymes do not show a typical Michaelis Menton constant. The classical hyperbolic curve is replaced by a sigmoid saturation curve.
Temperature
Heating increases molecular motion. Thus the molecules of the substrate and enzyme move more quickly and chances of their bumping into each other are increased. As a result there is a greater probability of a reaction occurring.
The temperature that promotes maximum activity is referred to as the optimum temperature. If the temperature is increased above this level, then a decrease in the rate of the reaction occurs despite the increasing frequency of collisions. This is because the secondary and tertiary structures of the enzyme have been disrupted, and the enzyme is said to be denatured. In effect, the enzyme unfolds and the precise structure of the active site is gradually lost. The bonds which are most sensitive to temperature change are hydrogen bonds and hydrophobic interactions.
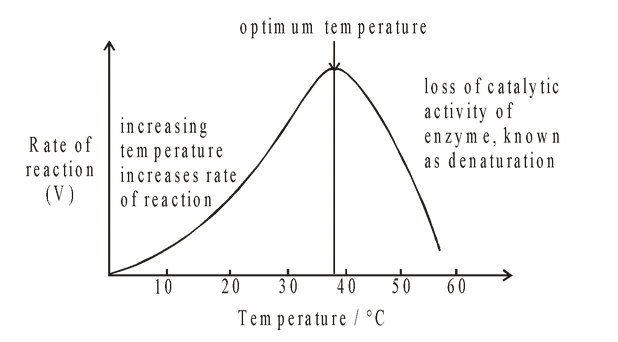
Most enzymes have a temperature optimum of about 37-40°C but enzymes with higher optima exist. For example the enzymes of bacteria living in hot springs may have an optimum temperature of 70°C or higher. Such enzymes have been used in biological washing powders for high temperature washes.
If temperature is reduced to near or below freezing point, enzymes are inactivated, not denatured. They will regain their catalytic influence when higher temperatures are restored.
The effect of temperature on the rate of a reaction can be expressed as the Temperature coefficient, Q10.
Q10 = rate of reaction at (x + 10) °C/rate of reaction at x °C.
Over a range of 0–40 °C, Q10 for an enzyme-controlled reaction is 2. i.e. the rate of an enzyme controlled reaction is doubled for every rise of 10°C.
pH
Under conditions of constant temperature, every enzyme functions most efficiently over a particular pH range, which is often the optimum pH at which the maximum rate of reaction occurs.
When the pH is altered above or below this value, the rate of enzyme activity diminishes.
As pH decreases, acidity increases and the concentration of H+ ions increases. This increases the number of positive charges in the medium.
Changes in pH alter the ionic charge of the acidic and basic groups and therefore disrupt the ionic bonding that helps to maintain the specific shape of the enzyme.
Thus the pH change leads to an alteration of enzyme shape, including its active site. If extremes of pH are encountered by an enzyme, then it will be denatured.
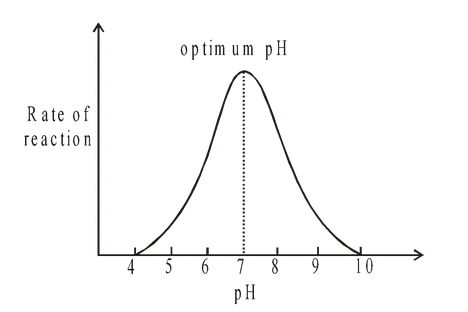
Fig. Effect of pH on the rate of an enzyme
