Mechanism of Enzyme Action
Enzymes of Class 11
Mechanism of Enzyme Action
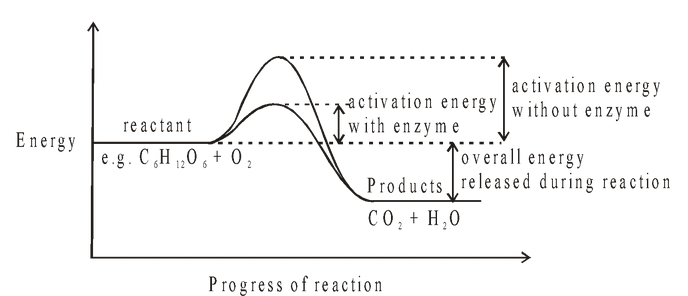
A little energy must be put in to get the reaction started. The energy is called the activation energy. It is the energy required to make the substances react. Enzymes, by functioning as
catalysts, serve to reduce the activation energy required for a chemical reaction to take place. They speed up the overall rate without altering, to any great extent, the temperature at which it occurs.
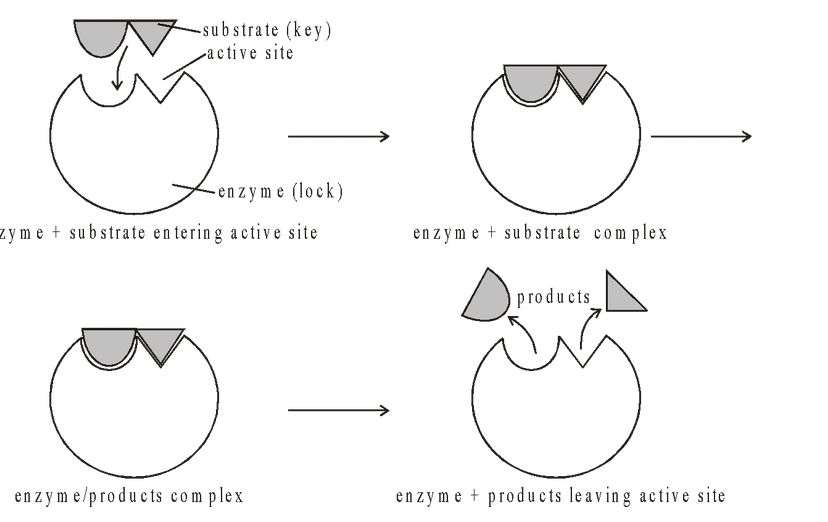
Lock and Key hypothesis :
Enzymes are very specific and it was suggested by Fischer in 1890 that this was because the enzyme had a particular shape into which the substrate or substrates fit exactly.
The substrate is imagined being like a key whose shape is complementary to the enzyme or lock. The site where the substrate binds in the enzyme is known as the active site which has the specific shape.
Most enzymes are far larger molecules than the substrates they act on and the active site is usually only a very small portion of the enzyme, between 3 and 12 amino acids.
The remaining amino acids, which make up the bulk of the enzyme, function to maintain the correct globular shape of the molecule which, is important if the active site is to function at the maximum rate.
Once formed the products no longer fit into the active sites and escape into the surrounding medium, leaving the active site free to receive further substrate molecules.
Induced fit hypothesis
In 1959 Koshland suggested a modification to the ‘lock and key’ model
Working from evidence that suggested that some enzymes and their active sites were physically rather more flexible structures, active site could be modified as the substrate interacts with the enzyme. The amino acids which make up the active site are moulded into a precise shape which enables the enzyme to perform its catalytic function most effectively.
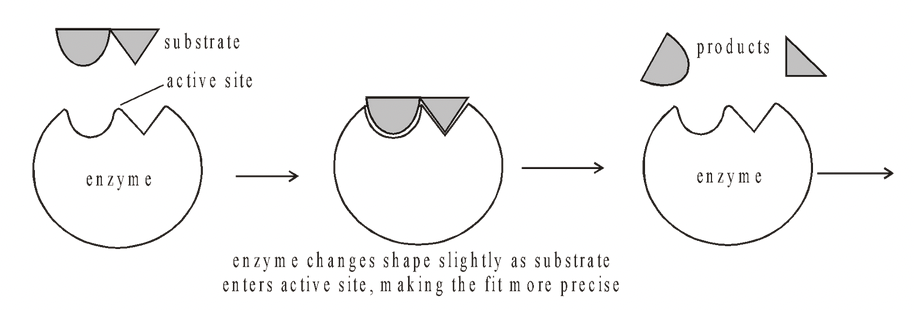
Koshland (1959) believes that each active site of an enzyme contains two regions, buttressing and catalytic. The buttressing region provides attachment site to the substrate molecules. For this it has various types of weak bonds or linkages. The catalytic group which lies at a distance from buttressing group is specialised to weaken bonds of reactant or substrate molecules through electrophilic and nucleophilic forces.
When the substrate molecules come in contact with buttressing group, the active site undergoes conformational change which brings the catalytic group opposite those bonds of the substrate which are to be weakened.
The rate of an enzyme reaction is measured by the amount of substrate changed, or amount of product formed, during a period of time.
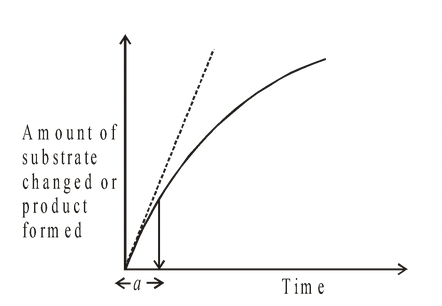
Fig. The rate of an enzyme controlled reaction
No. of substrate molecules converted per minute by one molecule of enzyme is known as turn over number. It depends on no of active sites, Precise collisions between reactant and enzyme and rate of removal of end products.
e.g. Carbonic anhydrase - Highest turnover no. i.e 36 million. Fastest enzyme hydrates 36 million CO2 molecules / minute into H2CO3 in RBC.
Lysozyme - Lowest turnover no i.e. 30. An anti-bacterial enzyme (actually is glycosidase that causes lysis of bacteria by hydrolysing glycosidic bonds. Tears are rich in lysozyme - discovered by Alexander Flemming.
- Introduction of Enzyme
- Properties of Enzymes
- Nomenclatures of Enzymes
- Chemical Nature of Enzymes
- Mechanism of Enzyme Action
- Factors affecting the Rate of Enzyme
- Enzyme Inhibition
- Equilibrium constant for inhibitor binding is called Dissociation constant.
- Economic Importance of Enzymes
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4
- Exercise 5









