Alkanes
Hydrocarbon of Class 11
Alkanes or paraffins are saturated hydrocarbons with general molecules formula CnH2n+2. Series of alkanes in which the members differ in composition from one another by ⎯CH2 group is known as homologous series, the individual members being known as homologues.
The nomenclature of alkanes is according to IUPAC rules.
eg. CH4 – Methane
METHODS OF PREPARATION
Alkanes are prepared by the following methods
(1) Reductions Methods:
Alkanes can be prepared by the reduction of various organic compounds as follows:
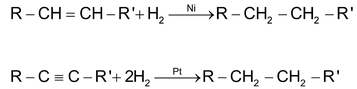
(a) By hydrogenation of unsaturated hydrocarbons
Alkanes are obtained by hydrogenation of unsaturated hydrocarbons (alkenes & alkynes) in presence of finally divided catalyst e.g. Ni, Pt, Pd etc.
The following reaction is known as Sabatier & Senderen’s reaction
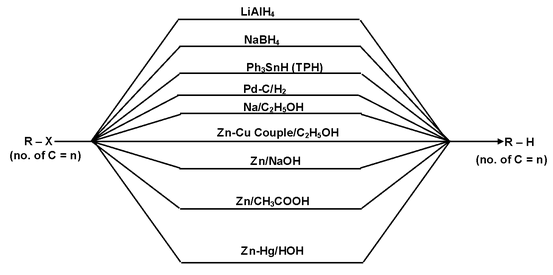
(b) By the reduction of Alkyl Halides:
Alkyl halides undergo reduction with nascent hydrogen to form alkanes.

By using following methods alkanes can be prepared:
Note:
(i) LiAlH4 is a very strong base and gives elimination reaction with 30 alkyl halides.
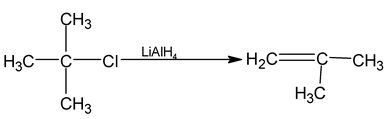
(ii) NaBH4 reduces only 20 and 30 alkyl halides
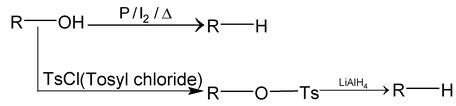
(c) By reduction of Alcohols: By following two methods, alkanes can be prepared from alcohols.
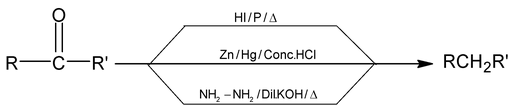
(d) By reduction of Carbonyl Compounds: By following three methods aldehydes & ketones are reduced to give alkanes.
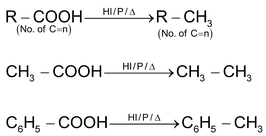
(e) By reduction of Carboxylic acid: Reduction by HI/P/Δ gives alkane.
(2) From Organometallic Compounds
Grignard’s reagents and alkyl lithium reacts with water and other compounds having acidic hydrogen to give hydrocarbon corresponding to the alkyl group of the organometallic compounds.
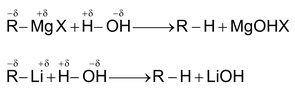
Grignard’s reagents reacts with alkyl halides to give alkanes
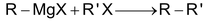 (Alkanes with higher number of carbons atoms)
(Alkanes with higher number of carbons atoms)
(3) From coupling reactions
(a) By Wurtz Synthesis:
Case 1: When both alkyl halides are same:
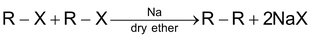
Case 2: When both alkyl halides are different:
RX + R′X  RR + R′R′ + RR′
RR + R′R′ + RR′
CH3CH2Br + CH3CH2CH2CH2Br 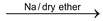 n butane + n – octane + n hexane
n butane + n – octane + n hexane
In this case a mixture of three alkanes is obtained & the separation of the mixture into individual member is not always easy, so it’s not a good method for the preparation of alkanes if both alkyl halides are different.
Note:
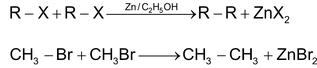
(i) CH4 can not be prepared by this method.
(ii) 30 alkyl halides do not give this reaction.
(iii) Alkenes are produced as by products.
(iv) This method is suitable for preparation of alkanes having even number of carbons.
(b) By Frankland’s reaction
Alkyl halides undergo coupling in the presence of Zn metal and ethyl alcohol.
(c) Corey – House Synthesis
Step (i): Alkyl halides react with lithium in dry ether to form alkyl lithium

Step (ii): This alkyl lithium reacts with CuI to give dialkyl lithium cuprate known as Gillman reagent

Step (iii): This further reacts with alkyl halides to give alkanes.
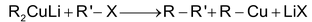
Some examples are
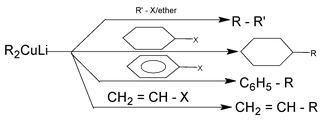
This method is suitable for the preparation of unsymmetrical alkanes of the type R⎯R′
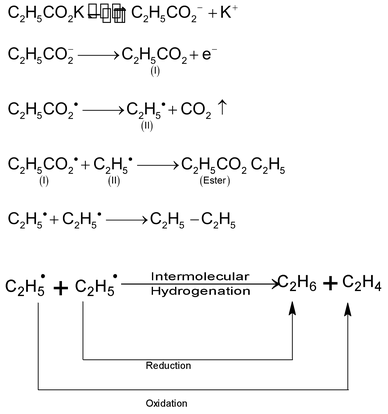
(d) By electrolysis of sodium or potassium salts of fatty acids (Kolbe’s electrolysis)
A concentrated solution of the sodium or potassium salt of a carboxylic acid or mixture of carboxylic acids is electrolysed.
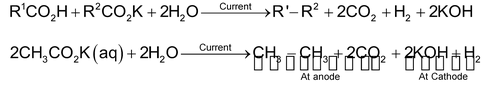
Esters, lower alkanes and alkenes are also obtained as side products.
(e) Hydroboronation
This process is used for preparation of long chain alkanes by coupling of alkyl boranes by means of silver nitrate in presence of NaOH at 250C. Diborane (B2H6) adds to an double bond forming trialkyl borane which on treatment with AgNO3/NaOH or acetic acid yields corresponding alkane.
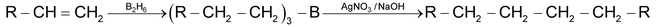
(4) Decarboxylation of acids
Sodium salts of fatty acids undergo decarboxylation when heated with soda lime.
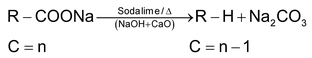
This reaction takes place as follows
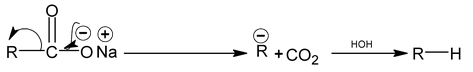
Note:
Sodium formate gives hydrogen gas instead of R – H.
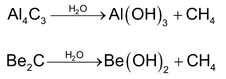
(5) Miscellaneous Methods
(a) Metal carbides (aluminium and beryllium carbides) on hydrolysis gives methane.
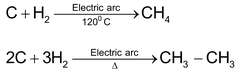
(b) Berthelot Synthesis can be used for the preparation of methane and ethane.
PHYSICAL PROPERTIES
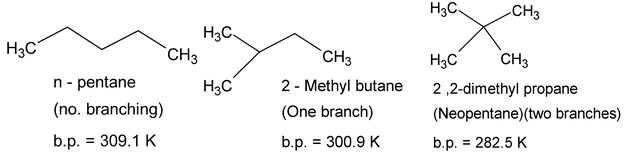
Principal intermolecular interactions between alkane molecules are London dispersion interactions. Due to weak forces, the C1 to C4 alkanes are gases, C5 to C17 are liquids, those with more than 18 carbon atoms are solids at 298K. All are colourless and odourless. Alkanes with straight carbon chains have higher boiling points than their isomers with branched chains.
CHEMICAL PROPERTIES
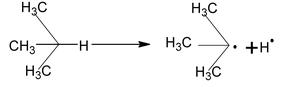
Alkanes are relatively stable to most of the common reagents at room temperature. The C ⎯ H and C ⎯C bonds in alkanes are almost non – polar and strong, and attacking reagents find no reaction sites to which they could be attached. Random collisions between them can occur but energy of collisions is too small to react. At high temperature energies of the collisions are sufficient for breaking C ⎯H and C ⎯C bonds resulting in the formation of free radicals.
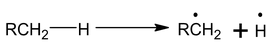
Stability of free radicals is in order
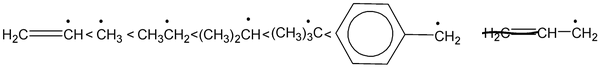
Substitution reactions of Alkanes
(A) Halogenation
It involves the replacement of 10, 20 & 30 H of alkanes by halogen (F, Cl, Br, I)
For a given type of abstraction of H (say 10) reactivity of halogen is in order:
F2 > Cl2 > Br2 > I2
For a given halogen, abstraction of 10, 20 and 30 H’s is in order:
10 < 20 < 30
For chlorination, reactivity of 10, 20, 30 H’s is in ratio:
1 : 2 : 3 : : 1 : 3.8 : 5
|
|
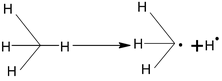
ΔH = 105 kcal mol−1 |
|
|
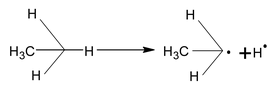
ΔH = 100 kcal mol−1 |
|
|
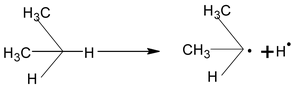
ΔH = 96 kcal mol−1 |
|
|
ΔH = 93 kcal mol−1 |
Bond energy of extraction of 10 H > 20 H > 30 H, hence the reactivity order is 10 < 20 < 30 H, hence, the stability order of alkyl radical free is

Mechanism

(i) Initiation:

(ii) Propagation:

(iii) Termination:

Based on relative reactivity of different types of H2, percentage of each isomer in the product mixture can be calculated. Consider the case of n – butane.
Relative amount = No. of equivalent hydrogen × reactivity

Total amount = 21.2
% of A = 6.0/21.2 x 100 = 28.3
% of B = 15.2/21.2 x 100 = 71.7
Bromine is less reactive with alkanes in comparison to chlorine, but bromine is more selective in the site of attack when it does react

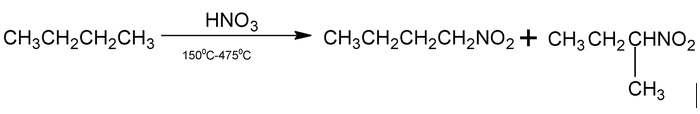
(B) Nitration
Replacement of H by a nitro group, ⎯NO2, is called nitration.
(C) Sulphonation
Replacement of H by a sulphonic acid group, SO3H is called sulphonation. Oleum is used as a source of (⎯SO3H).
RH + H2SO4/SO3 → RSO3H + H2SO4
(CH3)3CH + H2SO4/SO3 → (CH3)3CSO3H + H2SO4
Ease of replacement of H atoms is 30 > 20 > 10.