Factors Affecting Hb-O2 Dissociation Curve
Respiratory System of Class 11
Factors Affecting Hb-O2 Dissociation Curve
(i) Effect of Pco2 : If the Pco2 level is higher at the site of metabolism (tissue) the Hb releases O2 at faster rate hence the curve shifts to right hand side, this is called Bohr effect.
(ii) Effect of pH: The curve shifts to right hand side with the lowering pH i.e. increasing acidity, this also called as Bohr effect.
(iii) Effect of Temperature : Same as of Pco2.
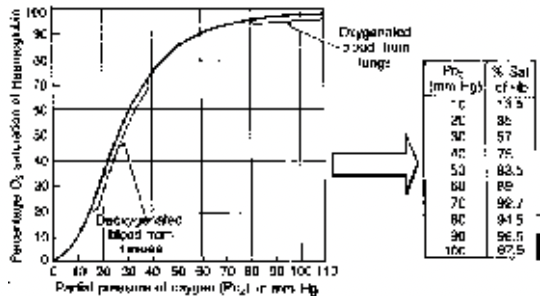
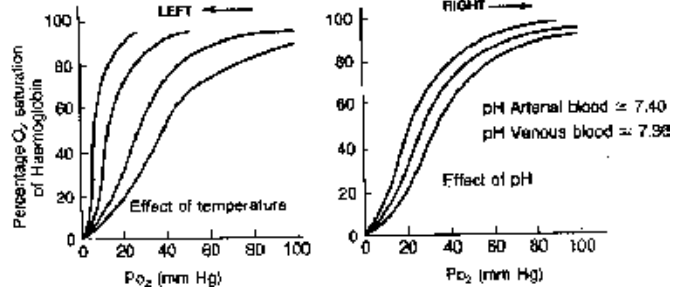
Fig. Effect of temperature and pH upon Haemoglobin - O2 dissociation curve
Transport of CO2
CO2 is highly soluble in water and hence transported in following three ways
(i) As simple solution of plasma = 5 – 7%
(ii) As carbonates/bicarbonates of Na and K = 65 – 85%
(iii) As carbamino compounds of protein (also Hb) = 10 – 20%
By dissolving in plasma (water), CO2 makes carbonic acid (H2CO3)
H2O + CO2  H2CO3
H2CO3
Carbonic acid is not allowed to pass in this form, as it will cause acidity of blood.
Minerals of plasma acting as buffer combine with it and form carbonates and bicarbonates These reactions are catalysed by enzyme carbonic anhydrase present in RBC.
H2CO3  H+ +
H+ + 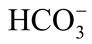
Na+ + H+ + 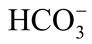
 Na2CO3 + H2O
Na2CO3 + H2O
Na+ + 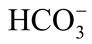
 NaHCO3
NaHCO3
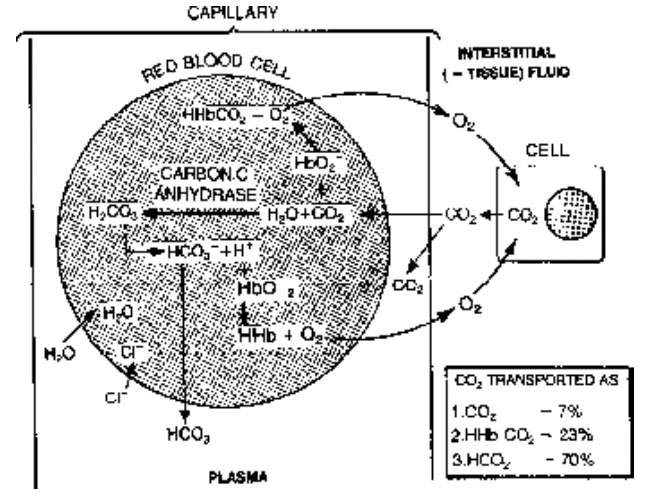
Fig. Transportation of CO2 by blood
As this reaction pathway is catalysed by enzyme carbonic anhydrase (present in RBC) CO2 is mainly (85%) transported as carbonates or bicarbonates and RBC has important role.
Protein also acts as buffer hence combines with CO2 to form carbamino compounds. Hemoglobin as protein acts in the same way
Pr + CO2  PrCO2 (Carbamino protein)
PrCO2 (Carbamino protein)
Hb + CO2  HbCO2 (Carbamino hemoglobin)
HbCO2 (Carbamino hemoglobin)
Hb has 25 - 30 times higher affinity for O2 than CO2.
Haldane Effect
It expressed in various following ways:
In presence of O2 the Hb does not combine with CO2; or releases CO2 immediately to combine with O2; or Hb has 20-30 times higher affinity for O2 than CO2; or HbO2 is acidic in nature and CO2 can not combine with it.
The binding of O2 with Hb tends to displace CO2 from blood. This is very important to promote the transport of CO2.
In tissue capillaries the Haldane effect causes increased pick up of CO2 after O2 splits from Hb and in the lungs it causes increased release of CO2 as the Hb combines with O2 here
Chloride shift or Hamburger’s shift
During CO2 transport the HCO3– level increases in the RBC hence negative charge increases here and to compensate this the Cl– ion comes out into plasma and enters back into RBC when HCO3 comes out of
it as KHCO3.
Carbon-monoxide poisoning
Hb has 300 times higher affinity for CO than O2 and combines to form carboxy haemoglobin (HbCO) a relatively more stable compound
Hb + CO  HbCO
HbCO
0.1% CO is sufficient to occupy all Hb in the blood to impair the O2 transport completely. This is why CO is a deadly poison as person dies of anoxia.
This generally happens when a person sleeps in closed room with a lamp burning inside. Also in the midst of thick vehicular traffic CO is released in the fume of half burnt fuel.
Ventilation decreases during sleep and sometimes it is so profound that it causes sleep apnoea even for longer (1 to 2 mts) time.
Mountain Sickness
When a person goes 8000 ft high from sea level i.e. on mountains, the related symptoms of sickness develop due to fall in pO2 and the less oxygenation of blood. These are giddiness,
headache, drowsiness, lattitude nausea, vomiting, mental fatigue, bluish tinge on skin, lips and nails.
Deep Sea Sickness
The deep sea divers generally face this problem when he goes down deep into sea and is lifted rapidly to the surface.
The phenomenon is as follows:
As the diver goes deep the water pressure rises which tends to collapse his lungs. Therefore, to prevent it he breaths compressed air at high pressure. But, this high pressure breathing
increases the partial pressure of gases in the alveoli. The rise of N2 (79% in air) pressure affects the body most as it diffuses and dissolves into blood and body fat. Due to this the diver loses strength, work capacity and feel drowsy.
Decompression Sickness (or Bend’s disease or Caisson’s disease or Dysbarism)
It is more severe than the former if the diver is lifted rapidly to the surface. Due to rapid fall in pressure nitrogen from the tissue evolves as gas bubbles which block the pulmonary vessels
causing loss of breath. By blocking the vessels in brain and spinal cord it causes dizziness, mental derangement, paralysis etc. It may block general circulation causing local pains and itching.
It is, therefore, advised that the divers should be lifted very slowly so that N2 is evolved slowly and removed from the body effectively without forming bubbles.
To avoid this problem deep divers use helium-oxygen mixture at high pressure.
Artificial breathing
In many conditions like drowning, electrocution, CO poisoning etc. the breathing may stop. Then artificial method is applied to ventilate the lung.
Mouth-to-mouth method
Patient is made to lie on the back, neck is raised by applying hand below it to open the air way; nostril is closed and then operator blows air into patients mouth from its mouth to fill the
lungs of patient. Next the air is allowed to pass out. This is repeated 10 -15 times per minute.









