Fundamental Forces In Nature
Units And Dimensions of Class 11
Force is a very common word which we normally come across in our daily life. We need force to push or pull or throw a body. Even we need it to deform or break the bodies. Sometimes, we experience force like when we are standing in a great storm, we experience the force exerted by wind. When we are sitting in a bus which is negotiating a turn, we experience an outward push. So, what is this force? Let us try to understand the concept of force in terms of physics.
At macroscopic level study of physics, we normally encounter different kinds of forces like gravitational force, muscular force, frictional force, contact force, spring force, buoyant force, viscous force, pressure force, force due to surface tension, electrostatic force, magnetic force, etc. whereas at microscopic level of study we come across nuclear forces, interatomic forces, intermolecular forces, weak forces, etc. Basically we classified them as fundamental interaction.
After analysing these various types of forces in nature, it was concluded that all the forces can be comfortably classified into four categories, which are known as fundamental forces in nature. They are
(1) Strong force (2) Electromagnetic force
(3) Weak force (4) Gravitational force
That means, any force other than the above four forces can be derived from these four basic forces. For example, elastic force or spring force arises due to the net attraction or repulsion between any two neighboring atoms of the spring. When it is elongated or compressed, attractive or repulsive forces produced between the atoms can be treated as the resultant of all electromagnetic forces between charged particles of an atom. Hence, this spring force is known as derived force and electromagnetic force which is the origin of this spring force is called fundamental force. Now, we will study about fundamental forces in brief.
Gravitational Force
|
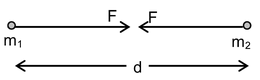
Newton discovered that any two bodies in universe attract each other. This force of attraction exists by virtue of their masses, and is known as gravitational force of attraction. He found that the gravitational force between two particle mass is directly proportional to their masses and is inversely proportional to the square of the distance between them. |
|
i.e. F = G 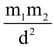 where ‘G’ is a Universal Gravitational Constant. This force is a universal force and is independent of any type of intervening medium between the two bodies. Though this is the weakest force in nature when compared to other types of fundamental forces, it plays vital role in governing the motion of planets around sun, natural satellites (like moon around earth), artificial satellites, etc.
where ‘G’ is a Universal Gravitational Constant. This force is a universal force and is independent of any type of intervening medium between the two bodies. Though this is the weakest force in nature when compared to other types of fundamental forces, it plays vital role in governing the motion of planets around sun, natural satellites (like moon around earth), artificial satellites, etc.
Electromagnetic Force:
The force of attraction or repulsion between any two charged particles is known as electrostatic force. If charges q1 and q2 are separated by a distance ‘d’ in air then the force of attraction or repulsion between them is given by F =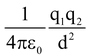 . This is called Coulomb’s law of electric forces.
. This is called Coulomb’s law of electric forces.
Charges in motion produce magnetic effects and a magnetic field gives rise to a force on a moving charge. In general electric and magnetic effects are inseparable and hence the name – electromagnetic force. This electromagnetic force between moving charged particles is comparatively more complicated and contains several other terms other than Coulomb’s force.
In atoms electromagnetic force between electrons and protons is responsible for several molecular and atomic phenomena. Apart from this it also plays vital role in the dynamics of chemical reactions, mechanical and thermal properties of materials, tension in ropes, friction, normal force, spring force, Vander Waals force .
Example: Let us consider a block which is placed on a horizontal surface of a table as shown in the figure. The table balances the weight (Mg) and exerts a force which comes from electromagnetic force between charged constituents of atoms or molecules of surface of block and that of the table. Thus a force called normal force acts on block.
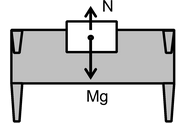
This electromagnetic force is a strong force when compared to the gravitational force. The electromagnetic force between two protons is 1036 times the gravitational force between them for any fixed distance.
Nuclear Force
We know that, in general, nucleus of every atom consists of two elementary particles called protons and neutrons. As neutrons are uncharged and protons are charged, the electric force of repulsion between protons will cause nucleus to break into fragments. But this is not happening, and also we know that nucleus of a non−radioactive element is a stable one.
That means there must be some other attractive force which is dominating coulombic force of repulsion between protons and keeping all the particles in nucleus together in stable condition as gravitational force can’t dominate electric force. That new force existing between any two nucleons and which keeps all the particles in nucleus bound together is known as nuclear force. This force is stronger than electromagnetic force and is a charge independent force. Range of these forces is very small and will be of the order of nuclear size (10−21 th portion of size of an atom).
Latest developments in physics revealed that this strong nuclear force is also not a fundamental force as protons and neutrons consist of still elementary particles called quarks. And according to this latest development quark – quark force is fundamental force of nature and nuclear force is a derived force. However the study of quark – quark force is out of the scope of this book and our curriculum.
Weak Nuclear Force
This force appears only in certain nuclear processes. A neutron can change itself into a proton by emitting an electron and another elementary particle called antineutrino simultaneously. This process is called 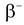 decay. Similarly a proton can also change into neutron by emitting positron and a neutrino. This process is called
decay. Similarly a proton can also change into neutron by emitting positron and a neutrino. This process is called 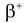 decay. The forces which are responsible for these changes are known as weak forces. These forces are weak in nature when compared to nuclear and electromagnetic forces but stronger than gravitational forces. The range of these weak nuclear forces is exceedingly small, of the order of 10−15m.
decay. The forces which are responsible for these changes are known as weak forces. These forces are weak in nature when compared to nuclear and electromagnetic forces but stronger than gravitational forces. The range of these weak nuclear forces is exceedingly small, of the order of 10−15m.
The following table gives us an overall idea about relative strengths and ranges of four fundamental forces.
|
Name |
Relative strength |
Range |
Operates among |
|
Gravitational force |
10−38 |
Infinite |
All objects in the universe |
|
Weak nuclear force |
10−13 |
Very short, within nuclear size (~10−15 metre) |
Elementary particles |
|
Electromagnetic force |
10−2 |
Infinite |
Charged particles |
|
Strong nuclear force |
1 |
Very short, within nuclear size (~10−15 metre) |
Nucleons |