Some Important Reactions Regarding Stoichiometry
Redox Reaction of Class 11
Some Important Reactions Regarding Stoichiometry
Effect of Heat on Carbonate and Bicarbonate
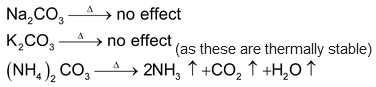
Other carbonates decomposes to give oxides and CO2
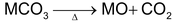
All bicarbonates decomposes to carbonates,CO2, HO2
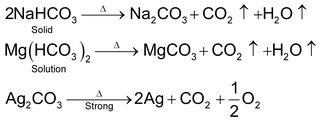
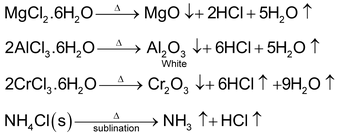
Effect of Heat on Chlorides, Bromides and Iodides
Generally these are not effected by heat.
Some halides of higher oxidation states changes into halides of lower oxidation state on heating.
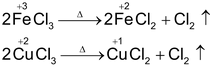
H2O and haloacids (HF, HCl, HBr,HI) such as
Effect of heat on Sulphates and Bisulphates
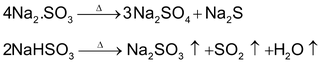
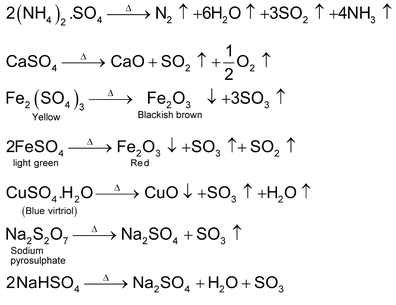
Effect of Heat on Sulphates, Pyrosulphates and Bisulphates
Sodium and potassium sulphates are thermally stable. However other sulphates, anhydrous or hydrous, decompose to give different products. Such as
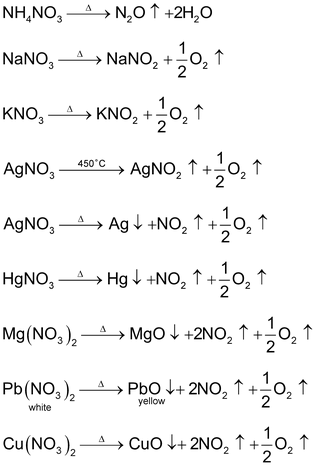
Effect of heat of Nitrates
They undergo thermal decomposition in following ways
Effect of heat on Oxide and Hydroxide
Hydroxide, and higher oxides and some metals undergo following change on heating strongly.
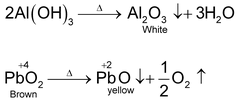
SOME OTHER IMPORTANT REACTIONS
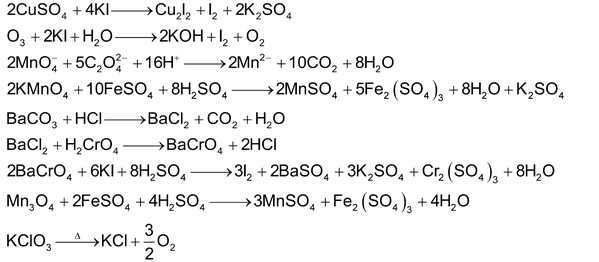
SOME IMPORTANT POINTS TO REMEMBER
If a salt of strong acid and strong base (e.g. NaCl) is present in the solution it will not react with any acid or base added to the solution.
If a salt of strong acid and weak base (e.g.NH4Cl) is present in the solution it will only react with strong base but it will not react with strong acid.

If a salt of strong base and weak acid (e.g CH3 COONa) is present in the solution it will react only with strong acid but it will not react with strong base.

- Introduction
- Electronic Concept Of Oxidation And Reduction
- Redox Reaction
- Oxidation Number Or Oxidation State
- Balancing Of Redox Reaction
- Ion Electron Method
- Volumetric Analysis
- Type Of Titrations
- Hardness Of Water
- Some Important Reactions Regarding Stoichiometry
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4









