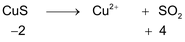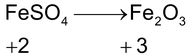Volumetric Analysis
Redox Reaction of Class 11
Important definitions:
(I)Molarity = No. of moles of solute/Volume of solution in K
(II)Normality = No. of gram equivalents of solute/Volume of solution in L
No. of gram equivalents of solute =Weight of solute/Equivalent weight
Equivalent weight = Molecular weight/'n'
It can be inferred that number of gram equivalents of a substance = n × number of moles and
Normality= ‘n’ × Molarity
It is extremely convenient to use the law of gram equivalents to solve problems based on chemical reactions. According to this law the ‘number of gram equivalents of all reactants are equal to each other in a reaction assuming none of them are in excess and is also equal to number of gram equivalents of all products’ assuming that all the reactants are undergoing reaction in the reaction.
We can use this law conveniently to solve problems without requiring to know much about the reactions. For this we need to have good understanding of the ‘n’ factor of a substance. ‘n’ factor is the valency factor or conversion factor.
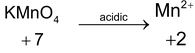
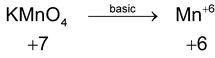
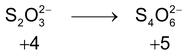
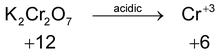
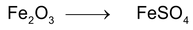
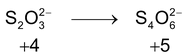
*n – factor of substance in redox reaction is equal to number of moles of lost or gain electron per molecule.
*n – factor of substance in non – redox reaction is equal to the product of displaced mole and its charge.
(1) Acids: The number of moles of replaceable H+ ions per mole of the acid e.g. for
HCl n = 1; H2SO4 n = 2; H3PO4 n = 3
(2) Bases: The number of moles of replaceable OH- ions per mole of the base e.g.
NaOH n = 1; Ba(OH)2 n = 2; Al(OH)3 n = 3
(3) Salts: Salts which reacts in such way that no atom in the salt undergoes change in oxidation state (oxidation state of an element in a molecule is the charge the element would have if all the bonds associated with the elements are assumed to be completely ionic.)
‘n’ factor = number of moles of cation in one mole of the salt × oxidation state of the cation.
∴ ‘n’ factor of MgCl2 = 1 × 2 = 2
(Note: whenever two substances react such that their ‘n’ factors are in the ratio of x : y, the molar ratio of these substances in the balanced chemical reaction would be in the ratio of y : x)
Examples
|
(i) |
n = 5 |
(vii) |
+6 +8 n = 2 |
|
(ii) |
n = 3 |
(viii) |
0 −2 n = 2 |
|
(iii) |
n = 1 |
(ix) |
n = 1 |
|
(iv) |
n = 6 |
(x) |
n = 6 |
|
(v) |
n =1 |
(xi) |
+5 +1 n = 4 |
|
(vi) |
+6+4 n = 2 |
(xii) |
n = 2 |
‘n’ factor for a redox reaction is the moles of electrons released or acquired by 1 mole of the reactant in the reaction.
Titration: The procedure for determining the concentration of a solution by adding its known volume to react completely with other solution of known strength whose volume justified by experiment.
Sodium of known strength is called standard solution are classified as
(i)Primary standards
(ii)Secondary standards
Substances preferred primary standards must have following characteristics:
- Easily available and easy to preserve (made stable) {resistant to moisture and air)
- Readily soluble in given solvent.
- The reaction with a standard solution should be of constant stoichiometry.
- Titration error should be negligible even at moderate concentration.
Some common examples with then use
Potassium hydrogen phthalate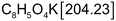 - Acid base
- Acid base
Anhydrous sodium carbonate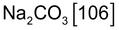 - Acid base
- Acid base
Potassium dichromate [294.19]-Redox
Arsenic oxide As2O3 [197.85]-Redox
Potassium iodate KIO3-Redox
Sodium oxalate Na2C2O4-Redox
EDTA [Na] [372.3]-Complexo method
The aim of titration is the addition of a quantity of standard and solution chemically equivalent to the quantity of unknown.
The completion of reaction is of ten indicated by a charge in colour of reaction mixture which may be either due to change in colour of reactant or a substance mixed externally known as indicator.
Further Reading :
- Introduction
- Electronic Concept Of Oxidation And Reduction
- Redox Reaction
- Oxidation Number Or Oxidation State
- Balancing Of Redox Reaction
- Ion Electron Method
- Volumetric Analysis
- Type Of Titrations
- Hardness Of Water
- Some Important Reactions Regarding Stoichiometry
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4