Hardness Of Water
Redox Reaction of Class 11
Hardness Of Water
Hard water with soap forms insoluble precipitates of calcium and magnesium salts of fatty acids.

Hardness of water has two types:-
Temporary hardness and Permanent hardness:
(i) Temporary Hardness: It is due the presence of bicarbonates of calcium and magnesium in water.
Removal process of temporary hardness:
(a) By boiling of water:
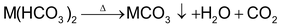
(b) Clarke’s process: By the addition of Ca(OH)2
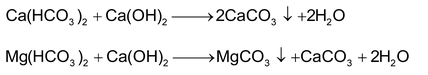
(c) By the addition of Na2CO3:

(ii) Permanent hardness: It is due the presence of chlorides and sulphates of Ca2+ and Mg2+.
Removal of permanent hardness:
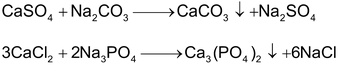
(a) By adding Na2CO3 or Na3PO4:
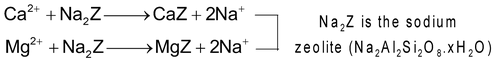
(b) Permuit process:
(c) Calgon (calcium gone) process:
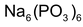 is called calgon which is written as
is called calgon which is written as 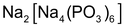
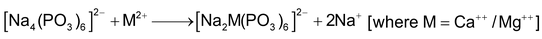
(d) Ion exchange resins process:
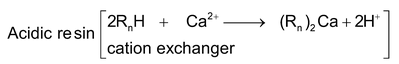
Degree of hardness: Degree of hardness defined as number of parts by weight of CaCO3 (or its equivalent quantities of other substance) present in million parts by weight of water.
Hardness of water 
Further Reading :
- Introduction
- Electronic Concept Of Oxidation And Reduction
- Redox Reaction
- Oxidation Number Or Oxidation State
- Balancing Of Redox Reaction
- Ion Electron Method
- Volumetric Analysis
- Type Of Titrations
- Hardness Of Water
- Some Important Reactions Regarding Stoichiometry
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4









