Electronic Displacement In Covalent Bonds
Fundamental of Organic Chemistry of Class 10
The following four types of electronic effects operates in covalent bonds
(i) Inductive effect
(ii) Resonance and mesomeric effect
(i) Hyperconjugation
Inductive effect
First let us look at the C −C bond in ethane. This C −C bond has no polarity because it connects two similar atoms. However, the C −C bond in ethyl chloride is polarized by the presence of the electronegative chlorine atom. This polarization is actually the sum of three effects. In the first of these, the C1 atom have been deprived of some of its electron density by the higher electronegativity of Cl. Secondly, the electron deficiency of C1 is partially compensated by drawing the C −C electrons closer to itself, resulting in polarization of this bond and a slight positive charge on the C2 atom. Thirdly, the polarization of the C −C bond causes a (slight) polarization of the three methyl C − H bonds. The effect of C1 on C2 is less than the effect of Cl on C1 atom.
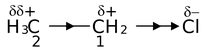
In addition to such inductive effect operating through the σ−bonds in a compound, an analogous effect can also operate either through the space surrounding the molecule or in solution via the molecules of solvent that surround it which is called field effect. A point of distinction between the two effects is that the inductive effect depends only the nature of bonds while the field effect depends on the geometry of the molecule. However, in many cases, it is not possible to distinguish inductive effect with this field effect. But in general, reference to an inductive effect is assumed to include any such field effect.
In ethyl chloride, Cl is said to have an electron withdrawing –I effect. There are other type of groups which exert electron releasing +I effect. Thus, the substituents or functional groups can be classified as electron-withdrawing (–I) and electron-donating (+I) groups relative to hydrogen.
The following is the order of decreasing inductive effects of most common groups.
+I groups : O-, CO2-, CR3, CHR2, CH2R, CH3, D
I groups : NR3+ , SR2+, NH3+, NO2, SO2R, CN, CO2H, F, Cl, Br, I, OAr, COOR, OR, COR, SH, OH, C ≡ CR, Ar, CH = CR2
For measurements of relative inductive effects, hydrogen is chosen as reference in the molecule CR3 – H. If the H atom in this molecule is replaced by X (an atom or group of atoms), the electron density in the CR3 part of the molecule is less than in CR3 – H, then X is said to have a –I effect (electron withdrawing or electron attracting). If the electron density in the CR3 part is greater than in CR3 – H, then X is said to have a +I effect (electron releasing or electron repelling).
All Alkyl groups exhibit +I effect when they are attached to an unsaturated or trivalent carbon (or other atom). Deuterium is also electron donating with respect to hydrogen. Keeping other things equal, atoms with sp bonding generally have a greater electron withdrawing power than those with sp2 bonding, which in turn have more electron-withdrawing power than with sp3 bonding. This accounts for the fact that aryl, vinylic and alkynylgroups exhibit –I effect.
Let us first compare the stabilities of following carbocations,
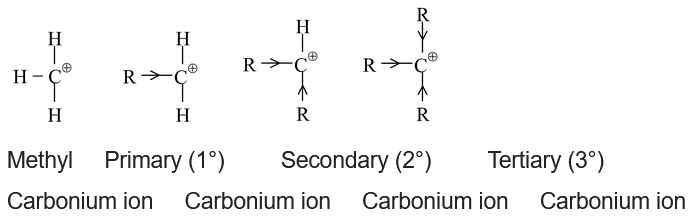
A primary (1°) carbocation is the one in which the carbon bearing the positive charge is attached to one carbon.
A secondary (2°) carbocation is the one in which the carbon bearing the positive charge is attached to two carbons.
A tertiary (3°) carbocation is the one in which the carbon bearing the positive charge is attached to three carbons.
RESONANCE & MESOMERIC EFFECT
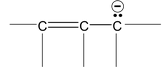
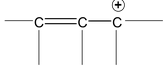
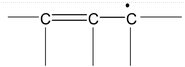
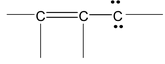
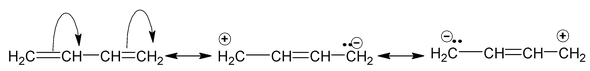
There are many organic molecules which can not be represented by a single lewis structure. In turn, they are assigned more than one structure called canonical forms or contributing of resonating structures. The phenomenon exhibited by such compounds is called resonance. For example, 1, 3 – butadiene has following resonance structure.
and canonical forms of vinyl chloride are
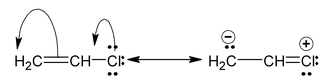
While drawing these canonical forms, the prime thing that has to be kept in mind is that the relative position of any of the atom should not change while we are allowed to change the relative positions of π - bonded electron pair or distribution of charge to other atoms. Also remember that it is not the case that some molecules have one canonical form and some have another form. All the molecules of the substance have the same structure. That structure is always the same all the time and is a weighted average of all the canonical forms. In real sense, these canonical forms have no expect in our imaginations. Now we are in a position to discuss about the conditions necessary for a compound to show resonance. The two essential conditions are
(a) There must be conjugation in the molecule. Conjugation is defined as the presence of alternate double and single bonds in the compound like
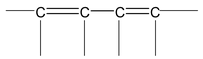
(b) The part of the molecules having conjugation must be essentially planar or nearly planar. The first condition of conjugation is not only confined to the one mentioned above but some other systems are also categorized under conjugation. These are
|
(i) |
|
(ii) |
|
(iii) |
|
|
(iv) |
|
(v) |
|
Resonance (mesomeric) effect is of two types
(i) If the atom or group of atom is giving electrons through resonance, it is called +R or +M effect For example,
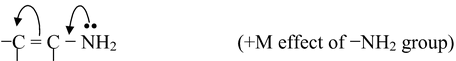
Other groups that shows +M effect are −NHR, −NR2, −OH, −OR, −NHCOR, −Cl, −Br, −I etc.
(ii) If the atom or group of atom is withdrawing electrons through resonance, it is called −R or −M effect. For example,
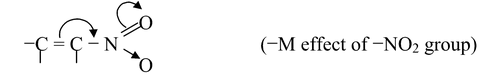
Other groups showing −M effect are −CN, −CHO, −COR, −CO2H, −CO2R, −CONH2, −SO3H, −COCl etc.
Now, let us consider resonance in nitrobenzene and its various canonical structures are
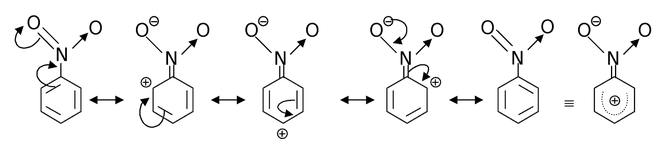
The –NO2 group in nitrobenzene has –M effect. In general, if any atom (of the group) attached to the carbon of benzene ring bears atleast one lone pair, then the group shows +M effect while if the atom (of the group) linked to the benzene carbon bears either a partial or full positive charge, then the group exhibits −M effect.
Example:1 Draw resonance structures for
(a) 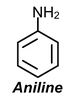
(b)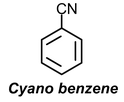
(c) 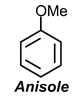
(d)
Solution
(a) 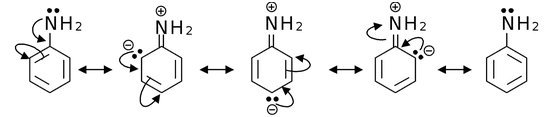
(b)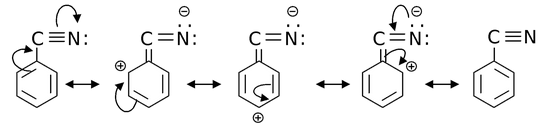
(c) 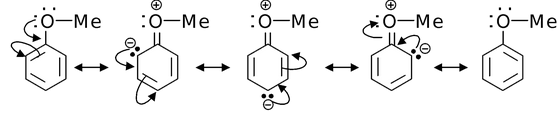
(d)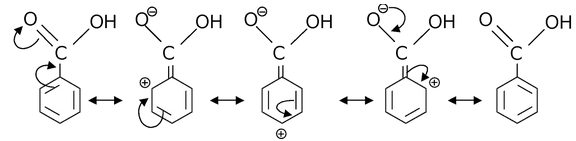
HYPERCONJUGATION
- It is delocalisation of sigma electrons.
- Also known as sigma-pi – conjugation or no bond resonance
- Hyperconjugation is a permanent effect
Occurrence
Alkene, alkynes
Free radicals (saturated type)carbonium ions (saturated type)
Condition
Presence of α–H with respect to double bond, triple bond carbon containing positive charge (in carbonium ion) or unpaired electron (in free radicals)
Example
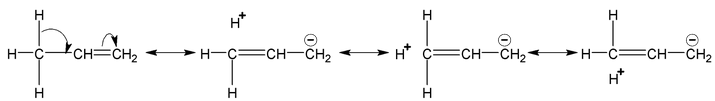
Note:
Number of hyperconjugative structures = number of α-Hydrogen. Hence, in above examples structures i,ii,iii,iv are hyperconjugate structures (4-structures).
When CH3 group is attached to an unsaturated atom or one with an unshared orbital, the canonical forms are drawn as
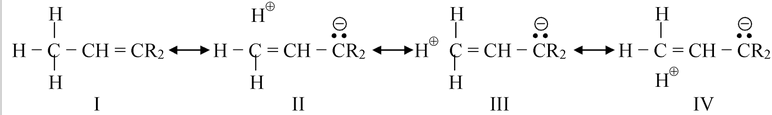
In such canonical forms there is no bond at all between the carbon and hydrogen but the hydrogen is not free as H+ because it will then contradict the condition of resonance, in which the relative position of the atoms in the molecule should not change. Thus, hyperconjugablehydrogens are not acidic in nature.
The effect of II, III and IV on the actual molecule is that the electrons in the C − H bond are closer to the carbon than they would be if II, III and IV did not contribute at all. For the other alkyl groups sequence –C2H5, –CH(CH3)2, –C(CH3)3, the hyperconjugation is further diminished because the number of C ⎯ H bonds decreases. Hence with respect to this effect, methyl is the strongest electron donor and t-butyl the weakest.
Stability of Intermediates
According to the laws of physics, the stability of a charged system is increased by the dispersal of charge. Any factor, which tries to spread out the positive chare of the electron deficient carbon of carbocation must stabilize it.
In the present case, it can be seen that greater the number of alkyl groups attached to the positive carbon of the carbocation, the more stable the carbocation will be. The reason is that electron donating groups (+I groups, alkyl groups in this case) partially compensates for the electron deficiency of the positive carbon of the carbocation. Thus, the order of stability of carbocations would be
Tropyliumcation> (C6H5)3C⊕> (CH3)3C⊕> CH2 = CH −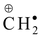 > (CH3)2CH⊕>
> (CH3)2CH⊕>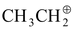 >CH3⊕
>CH3⊕
Triphenyl Benzyl Allylcation Isopropyl Ethyl Methyl
methylcation cation cation cation cation
Secondary > Primary > Methyl Carbocation
Explanation: Tropyliumcation, (C6H5)3C⊕, CH2 = CH − 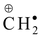 stabilized by resonance only where as (CH3)3C⊕ , (CH3)2CH⊕ and
stabilized by resonance only where as (CH3)3C⊕ , (CH3)2CH⊕ and 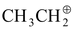 Carbocation are stabilized by both inductive and hyper conjugation effect.
Carbocation are stabilized by both inductive and hyper conjugation effect.
The presence of electron withdrawing group (−I group) would decrease the stability of carbocation.
Similarly, the stability of a free radical can be increased by the presence of +I groups and decreased by the presence of −I groups. Thus, the order of stability of free radicals would be
Benzyl >Allyl > 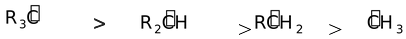
3° free radical 2° free radical 1° free radical methyl free radical
Explanation: Benzyl and allyl radicals are stabilized by resonance whereas alkyl radicals are stabilized by hyperconjugation.
In carbanions, the presence of electron donating groups (+I groups) would destabilize it while presence of electron withdrawing groups (−I groups) would stabilize it. Thus the order of stability of carboanions would be
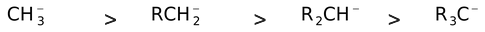
methylcarbanion 1° carbanion 2° carbanion 3° Carbanion
Phenyl > Methyl > Ethyl > n−propyl > isopropyl > isobutyl
Explanation: All carbon atoms of phenyl anions are sp2hybridised whereas other anions of the above order are in sp3hybridised state.