
Reaction Intermediates
Fundamental of Organic Chemistry of Class 10
Most of organic reactions occurs through the involvement of certain chemical species. These are generally short lived (10-6 seconds to a few seconds) and highly reactive and hence can not be isolated. These short lived highly reactive chemical species. Through which the majority of the organic reactions occur are called reactive intermediates. These intermediates are detected by spectroscopic methods or trapped chemically or their presence is confirmed by indirect evidence. On the other hand, synthetic intermediate are stable products which are prepared isolated and purified and subsequently used as starting materials in a synthetic sequence.
1.CARBOCATIONS
Carbocations are the key intermediates in several reactions and particularly in nucleophilic substitution reactions and electrophilic addition reaction.
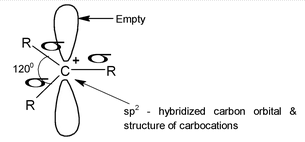
(a)Structure:
Generally in the carbocations the positively charged carbon atom is bonded to three others atoms and has no nonbonding electrons. It is sp2 hybridized with a planer structure and bond angles are of about 1200. There is a vacant unhybridised p orbital which (e.g in the case of CH+3) lies perpendicular to the plane of C ⎯ H bonds.
2.Free Radicals
Their formation is initiated mostly by ultra−violet light. For example,
Cl−Cl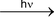 2Cl
2Cl
R − H + Cl → R + HCl
This alkyl group bearing an odd unpaired electron is called alkyl radical. The radicals are electron deficient and paramagnetic in nature. A free radical has carbon in the sp2 hybridised state, thus exhibit planar (flat) structure.
3.CARBANIONS
Chemical species bearing a negative charge on carbon and possessing eight electrons in its valence shell are called carbonions. These are produced by heterolylic cleavage of covalent bonds in which the shared pair of electrons remain with the carbon atom.
(a)Structure:
A carbanion posses an unshared pair of electron and thus represents a base. The best likely description is that the central carbon atom is sp3 hybridized with the unshared pair occupying one apex of the tetrahedron. Carbonions would thus have pyramidal structures similar to those of amines. It is believed that carbanions undergo a rapid interconversion between two pyramidal forms.
There is evidence for the sp3 nature of the central carbon and for its tetrahedral structure.
At bridgeheads carbon does not undergo reaction in which it is converted to a carbocation. However, the reactions which involve carbanions at such centre take place with ease, and stable bridgehead carbanion are known

