
Hydrides
Hydrogen of Class 11
Dihydrogen combines with a number of elements to form binary compounds called hydrides. Their general formula being MHX where M represents the element and x the number of hydrogen atoms.
Depending upon the physical and chemical properties, the hydrides have been divided into the following three broad categories:
1. Ionic or salt-like or saline hydrides
2. Metallic or Interstitial hydrides
3. Molecules or Covalent hydrides
Saline Hydrides or Ionic hydrides
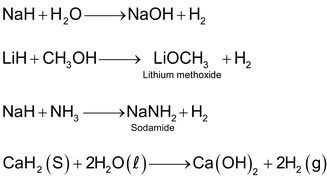
These are binary compounds of hydrogen and elements which are more electropositive than hydrogen such as alkali metals, alkaline earth metals (except Be), etc. Saline hydrides are formed by the transference of electron from metal to hydrogen. Some common examples of this category are: LiH, NaH,CaH2,CrH2 etc. The general characteristic of these hydrides are as follows:
(i) They are crystalline solids having white or greyish colour.
(ii) They have high melting and boiling points.
(iii) They have high density and high heat of formation.
(iv) They conduct electricity in molten state liberating dihydrogen gas at anode which confirm the presence of hydride (H-) in then.

At anode:

(v) They react vigorously with water and other protonic solvents such as ethanol and ammonia to liberate dihydrogen gas. Thus they act as strong bases.
Covalent Hydrides or Molecular Hydrides
These are binary compounds of hydrogen and elements of comparatively high electronegativity such as p- block elements. In these hydrides, H atoms are bonded to the other atoms by covalent bonds. Some examples of covalent hydrides are, HCl,H2O, PH3, NH3 etc. The general formula of covalent hydrides can be written as XH(8 - n) where n is the number of outershell electrons of X atom. However, elements of group 13 are exception to this formula. The elements of group 13 such as B, Ga form polynuclear hydrides which are electron deficient compounds. B2H6,Ga3H2 etc, are some examples. Some of the general characteristic of covalent hydrides are as follows:
(i) These hydrides consist of individual covalent molecules with relatively weak interparticle forces (Vander waal’s force of attraction). Hence they generally soft, with low melting and boiling points.
(ii) They are poor conductors of electricity.
(iii) Being covalent in nature, they are more soluble in organic solvents.
(iv) They undergo thermal decomposition into their respective elements.

(v) They covalent in nature, they are more soluble in organic solvents.
(vi) Some of them react with water to liberate H2.
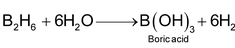
(vii) Along any given row of periodic table, the covalent hydrides become increasing acidic in moving from left to right.
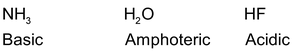
Interstitial Hydride or Metallic Hydrides
These are binary compounds of hydrogen and transition elements.
These hydrides are generally formed by the
(a) transition metals of group 3, 4, 5 of d- block;
(b) Cr metal of group 6 and
(c) f – block elements.
It may be noted that elements of group 7, 8, 9 of d – block do not form hydrides at all. This inability of metal, of group 7, 8, 9 of periodic table to form hydrides is referred to as hydride gap of d – block.
In these compounds H atoms are supposed to occupy interstitial position in the metal lattices. Some scientists consider these compounds as simply solid solutions of hydrogen. The composition of these hydrides may not correspond to simple whole number ratio and therefore, they are also called non-stoichimotric hydrides. Their composition is also found to vary with the conditions of temperature and pressure. Some examples of interestial hydrides of elements of group 3 to 5 are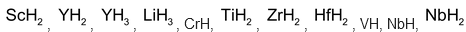 , etc.
, etc.
Some examples of non-stoichimetric hydrides are
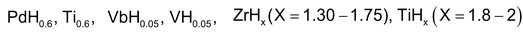 , etc.
, etc.
Some general characteristics are as follows:
(i) They are generally powders or brittle solids having dark or metallic appearances.
(ii) They are good conductors of electricity. The conductivity, however, decreases with increase in temperature.
(iii) They have high thermal conductivity.
(iv) Most of these hydrides are harder than parent metals.
(v) They generally undergo reversible decomposition into H2 gas and metal.
Besides three main categories of hydrides some other types of hydrides are also known. Two of these are described as follows:
(a) Polymeric Hydrides
They are formed by the elements having electronegativity range between 1.4 and 2.0. They consist of molecules held together in two or three dimensions by hydrogen bridges. Some common examples are
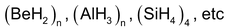
They are amorphous solids and stable up to 525 K. Above this temperature they begin to evolve hydrogen gas.
(b) Complex Hydrides
These are the compounds which contain hydride ions (H-)co-ordinated to metal atom ions. Some common examples are LiAIH4 (lithium aliminium hydride), NaBH4(sodium borohydride) etc. They are generally very good reducing agents.
