
Introduction
Hydrogen of Class 11
Hydrogen is the first element in the periodic table and is also the lightest element known. Its atomic form exists only at high temperatures. In the normal elemental form, it exists as a diatomic molecule, i.e.H2.
Unique Position of Hydrogen in the periodic table
A proper position could not be assigned to hydrogen either in the Mendeleev’s periodic table or Modern periodic table because of the following reason:
In some properties, it resembles alkali metals and in some properties it resembles halogens. So hydrogen can be placed both in group 1 and group 17 with alkali metals and halogen respectively.
Resemblance with alkali metals
1. Electronic configuration.
Hydrogen contains one electron in the valence shell like alkali metals
H : 1s1
Li : [He]2s1
Na : [Ne]3s1
K : [Ar]4s1
Rb : [Kr]5s1
2. Electropositive Character
Like alkali metal, hydrogen also loses its only electron to form hydrogen ion, i.e, H+
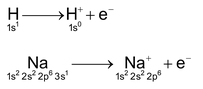
3. Oxidation state
Like alkali metals, hydrogen exhibits an oxidation state of +1 in its compounds.

4. Reducing agent
Alkali metals act as reducing agents because of their tendency to lose valence electron. Hydrogen is also a very good reducing agent as evident from the following reactions:
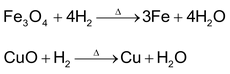
5. Combination with electronegative elements
Just like alkali metals hydrogen combines with electronegative elements such as halogen, oxygen, sulphur, etc to form compounds with similar formulae
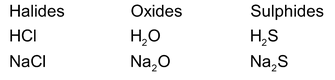
Difference from Alkali Metals
1. Ionization enthalpy
Ionization enthalpy of hydrogen (1312 kJ mol−1) is very high in comparison with the ionization enthalpy of alkali metals.
2. Existence of H+
It has been established that H+ ion does not exist freely in a aqueous solution. This is because of the fact that has a very small size (≈ 1.5 x 10-3pm) as compared to normal atomic and ionic size (which range from 50 to 220 pm). Thus it exists in aqueous solution in the form of hydrated proton with a formula,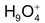 . However, for the sake of simplicity hydrated proton is represented by hydronium ion,H3O+.
. However, for the sake of simplicity hydrated proton is represented by hydronium ion,H3O+.
On the other hand, the alkali metal ions mostly exist as hexahydrated ions.
3. Difference in halides
Hydrogen halides are different from the halides of alkali metals although they have similar molecular formulae. For example
(i) Pure HCl is a covalent compound while NaCl is an ionic compound.
(ii) HCl is a gaseous compound while NaCl is a solid at ordinary temperature.
Resemblance With Halogens
1. Electronic configuration
Just like halogens, hydrogen needs one electron to attain the configuration of nearest noble gas.

2. Atomicity
Like halogens, hydrogen also exists in a diatomic state. The atomicity of hydrogen as well as halogens is two.
3. Electrochemical nature
During electrolysis of LiH, CaH2, etc, in molten state hydrogen is evolved at the anode indicating its electronegative nature. In this respect, hydrogen shows resemblance with halogens which are also liberated at the anode during electrolysis.
4. Oxidation state
Just like halogens, hydrogen also exhibit state of -1 in some of its compounds such as metal hydrides. 
5. Combination with alkali metals
Just like halogen, hydrogen also combines with alkali metals to form salts with similar formulae.

6. Combination with non-metals
Just like halogens hydrogen also react with non-metals such as carbon, silicon, germanium, etc, to form covalent compounds.
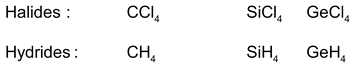
7. Ionization energy
Ionization energy of hydrogen is comparable to the ionization energies of halogens as shown below:
Element : H F Cl Br
Ionization energies (kj mol-1) : 1312 1681 1255 1121
Difference from halogens
1. Less tendency of hydride – formation.
Although hydrogen forms hydride ion (H−) like halogens, yet its tendency to form hydride ion is very less in comparison with the halogens. It is quite clear from the fact that halogens form halides with very large number of metals but hydrogen form hydrides with only a small number metals like sodium and calcium, etc.
2. Absence of unshared electrons
There is no unshared pair of electron in hydrogen molecule (H2) whereas halogen molecules have six unshared electron pairs as shown below:
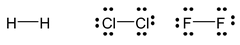
3. Nature of oxides.
The oxides of halogen are acidic in nature whereas oxide of hydrogen is neutral

4. Nature of compounds
The compounds of hydrogen with halogens, i.e. hydrogen halides (HF, HCl, HBr, HI) are low boiling covalent compounds whereas alkali metal halides (LiF, NaCl, KBr, CsI) are high melting ionic solids.
Conclusion
From the above discussion, it is quite evident that there is a marked resemblance in the properties of hydrogen with alkali metals as well as with halogens. Therefore, it is very difficult to place it either with the elements of group 1 or those of group 17. In other words position of hydrogen in the periodic table is anomalous. It is due to this reason hydrogen is some times called rogue element.
