
Concept Of Limiting Reagent
Some Basic Concept Of Chemistry of Class 11
Concept Of Limiting Reagent
Limiting Reactant
The reactant which is totally consumed during the course of reaction and when it is consumed reaction stops.
The concept of limiting reactant is applicable to reaction other than monomolecular i.e., when more than one type reactant involved.
For example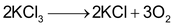 . These is no limiting reactant.
. These is no limiting reactant.
To determine the limiting reagent amount of all reactants and mole ratio of reactants must be known. If the ratio of moles of reactant A with respect to reactant B is greater than the ratio of the moles of A to moles of B for a balanced chemical equation than B is the limiting reactant.
All other terms like left (unused) mass of other reactant, amount of formed product can be known stoichiometrically by knowing the amount of limiting reactant.
Method of Expressing Concentration of Solution
- Molarity (M): The molarity of a solution is the number of moles of solute present in one litre (1dm3) of the solution
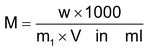
- The molality (m): The molality is the number of moles of solute present in one Kg of solvent
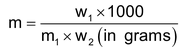
- Relation between molarity and molality
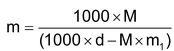 (Where d = density of solution)
(Where d = density of solution)
Parts per million parts (ppm): For every dilute solution, i.e., when a very small quantity of a solute is present in large quantity of a solution, the concentration of the solute is expressed in terms ppm. It is defined as the mass of the solute present in one million (106) parts by mass of the solution. Thus for a solute A,
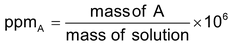
The pollution of the atmosphere is also reported in ppm but it is expressed in terms of volumes rather than masses, i.e. volume of the harmful gas (e.g. SO2) in cm3 present in 103 cm3 of the air.
(ii) Relationship between molarity (M) and molality (m): Molarity M means M moles of solute are present in 100 cc. of the solution. If density of the solution is d g/cc, mass of solution = 1000 f grams. Mass of solute = MM2g (M2 is mol mass of solute).
Hence mass of solvent = 1000d – MM2g.
∴ Molality (m) = 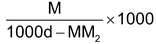
= 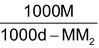
Thus m = 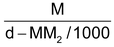
or 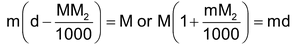
or 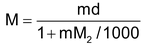
(iii) Relationship between molality (m) and mole fraction (x2): Molality (m) means m moles of the solute in 1000 g of the solvent = 1000 / M1 moles (M1 = mol of mass of the solvent). Hence
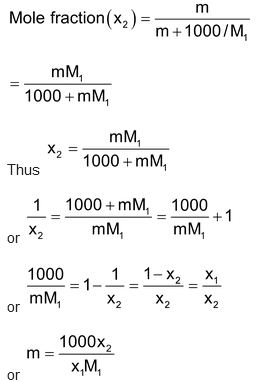
(iv) Relationship between molarity (M) and mole fraction (x2): Referring to calculations in (ii) above,
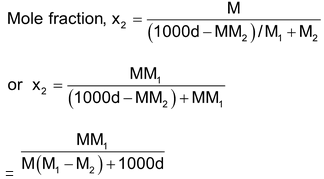
Thus x2 = 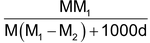
Or rearrangement, we get
Or 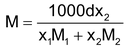
Note:
If molarity (M) is in moles / litre and density d is kg/litre and molality m is in moles / kg of the solvent, 1000 will be replaced by 1 in the above formulae.
