Electric Charge and Fields NEET Notes : Electric Charge and Fields are one of the most important topics in physics for NEET preparation. This chapter is part of the electrostatics unit and lays the foundation for understanding many other concepts in physics. A clear understanding of this topic is essential for solving numerical problems and answering theory-based questions in the NEET 2025 exam .
Also Check:
Electric Charge and Fields NEET Notes Overview
Electric Charge and Fields NEET Notes provide a comprehensive understanding of Electric Charge and Fields, a foundational topic in the Physics section crucial for NEET aspirants. It begins with the properties of electric charge and the methods of charging, offering insights into conduction, induction, and friction. The chapter progresses to explain Coulomb's Law and the superposition principle, which are essential for calculating forces between multiple charges. The concept of the electric field and its representation through field lines is explored in detail, along with the behavior of electric dipoles in these fields.



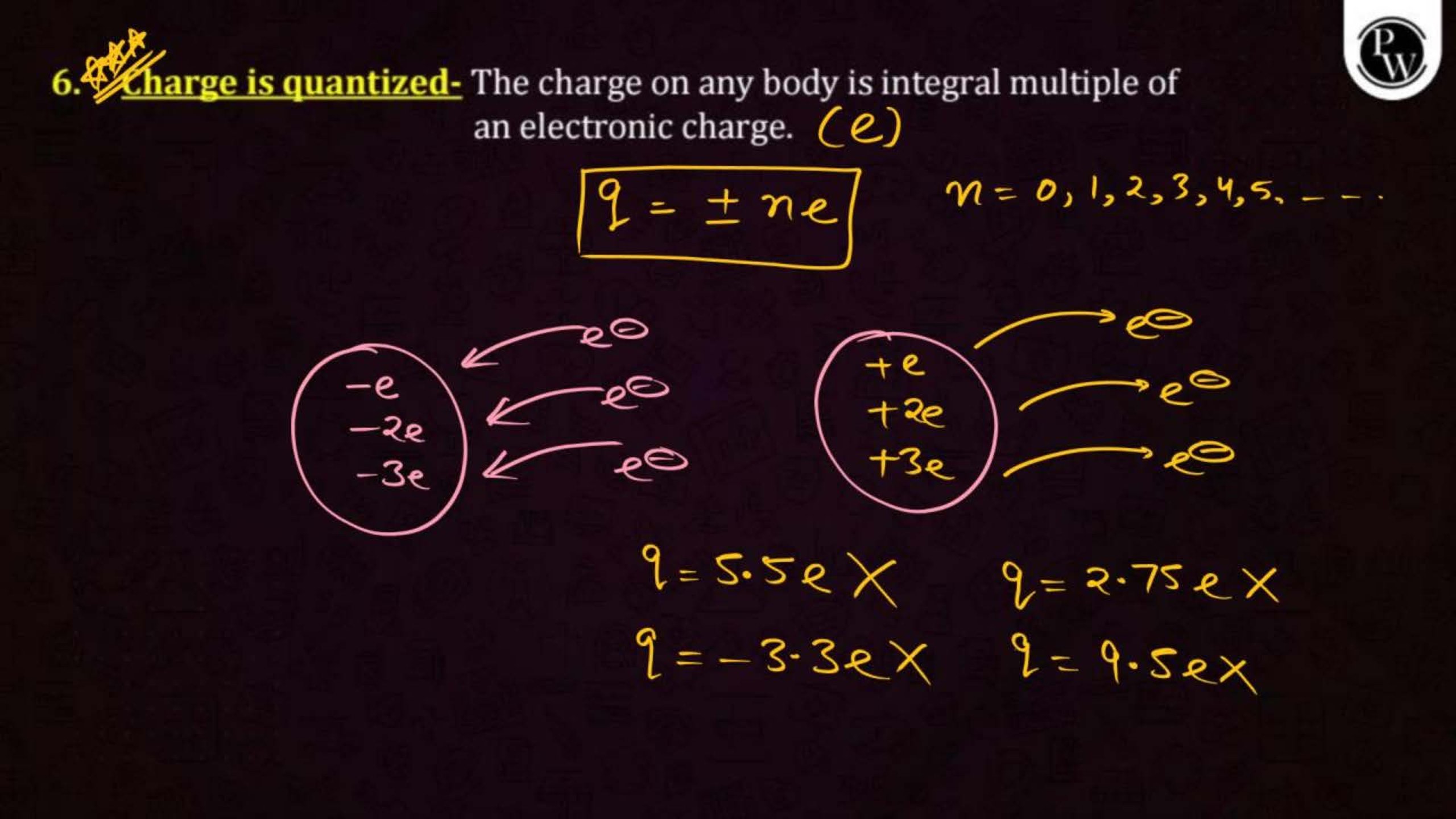


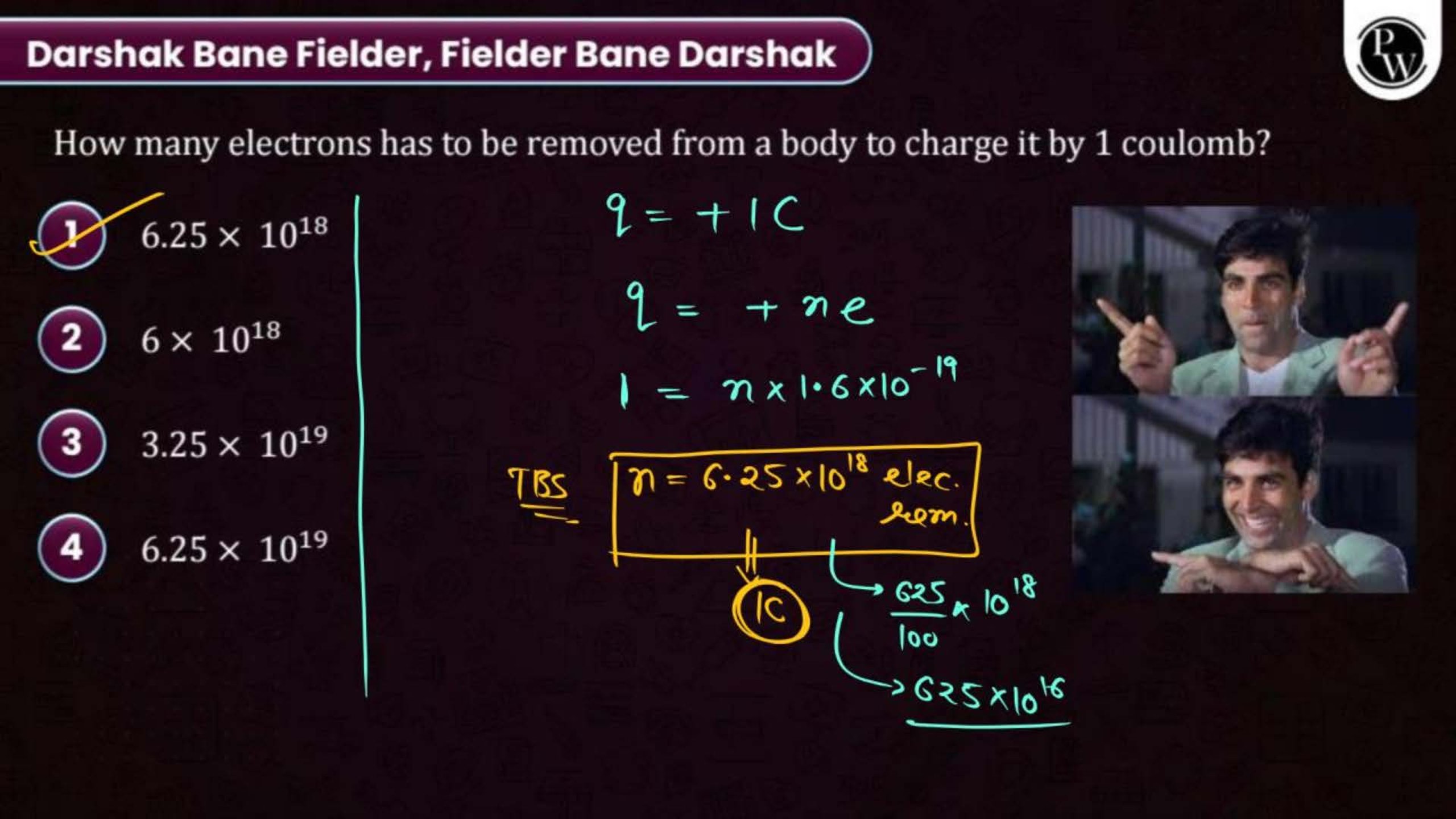
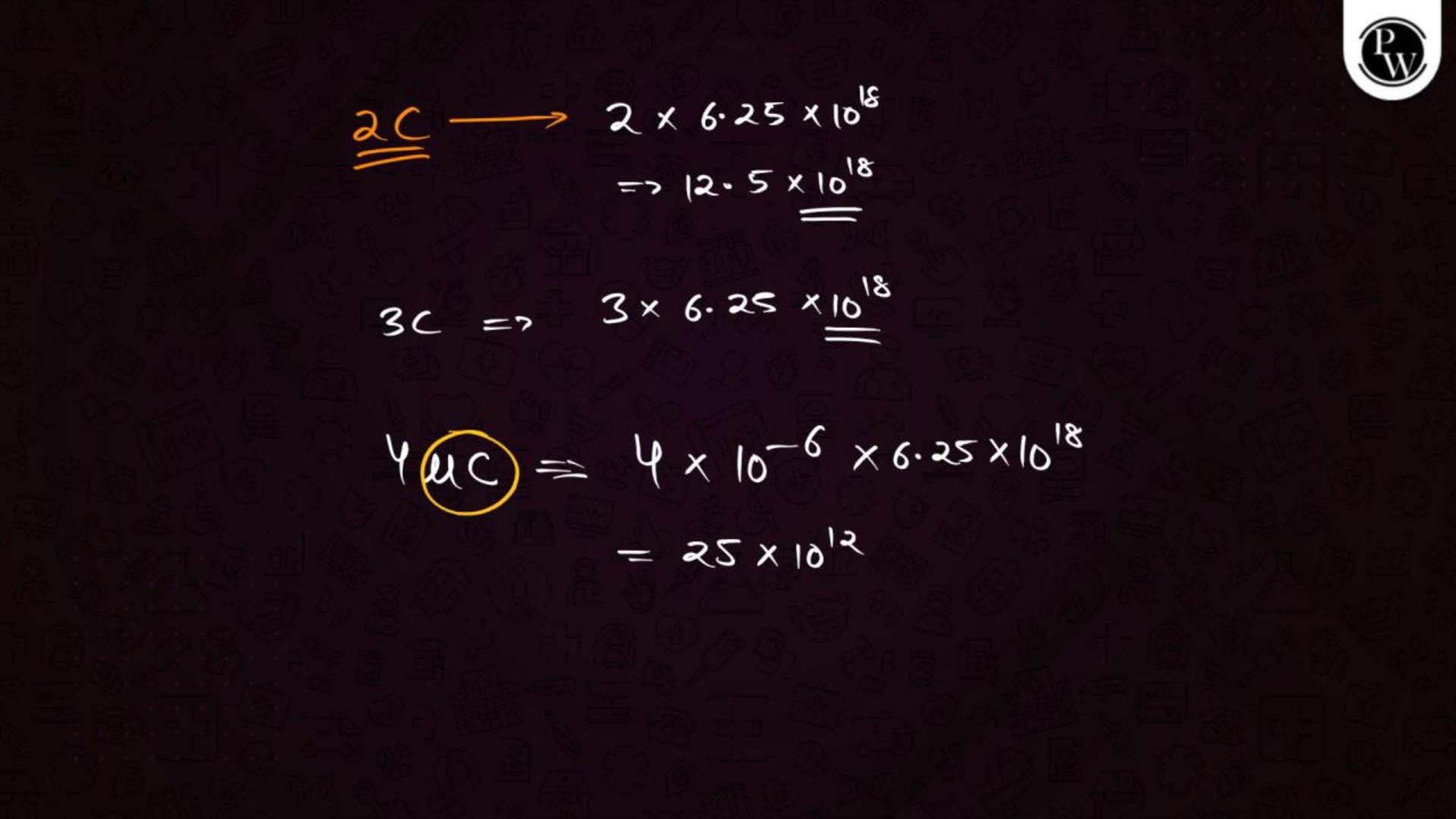
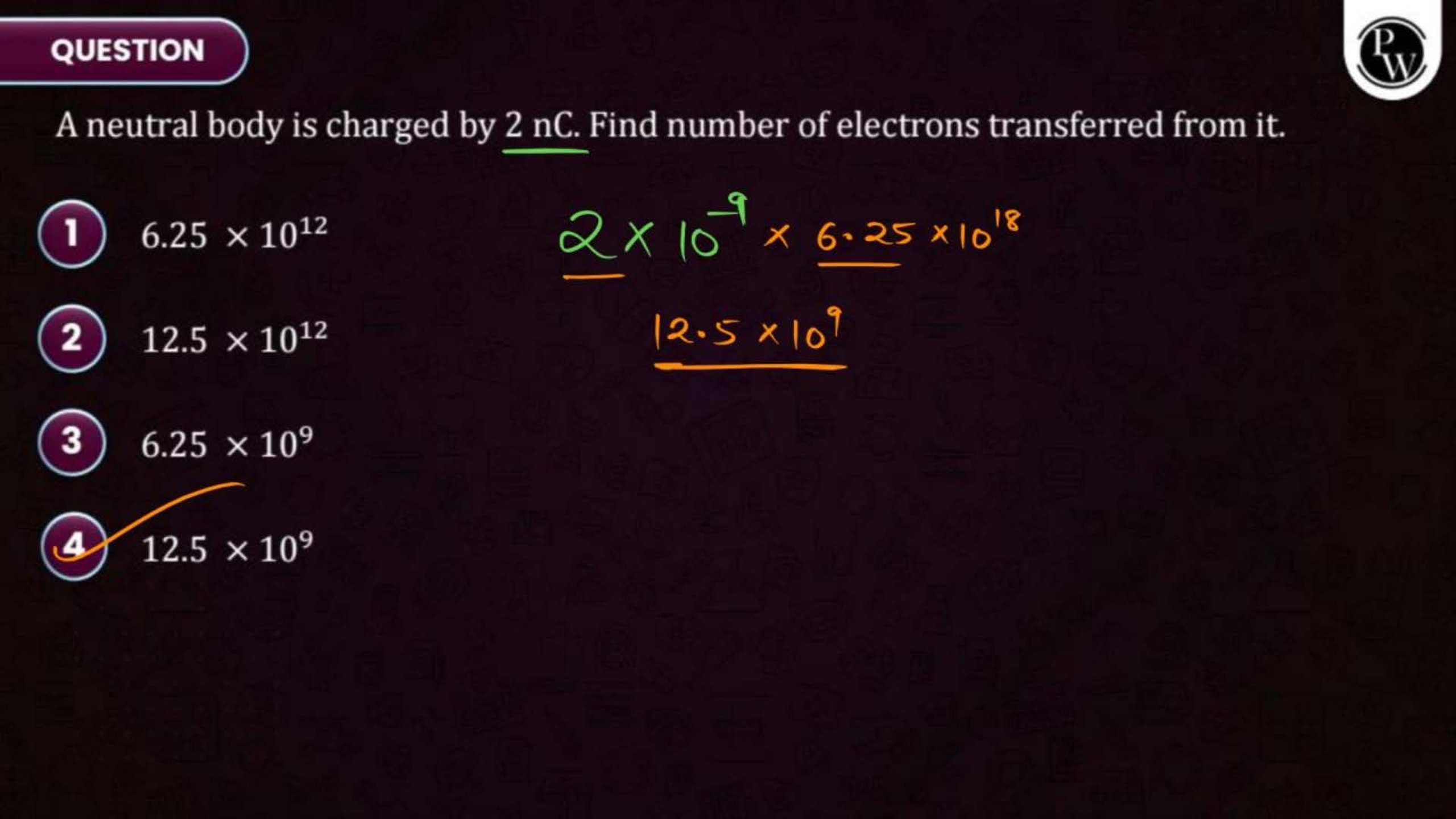
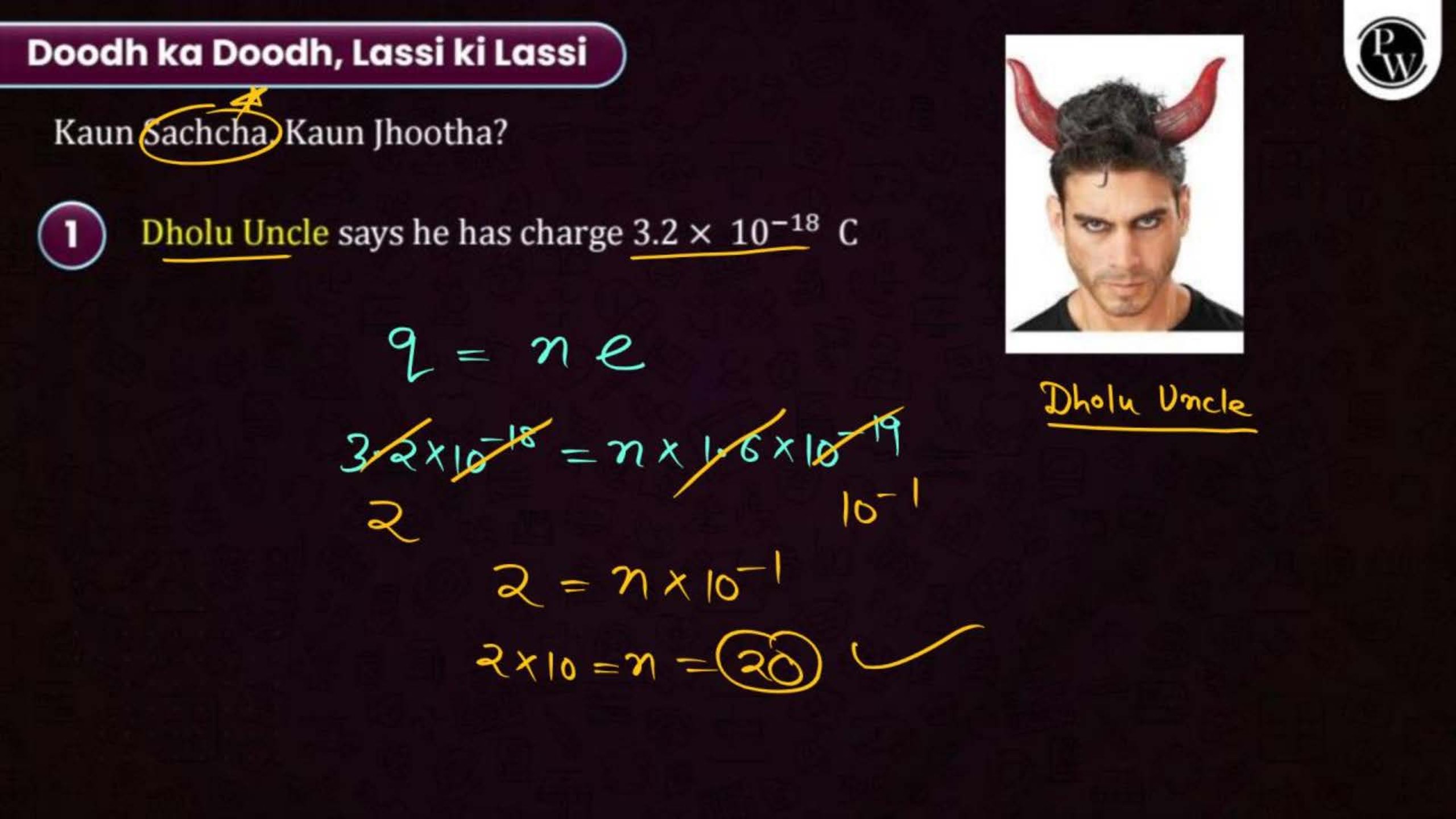
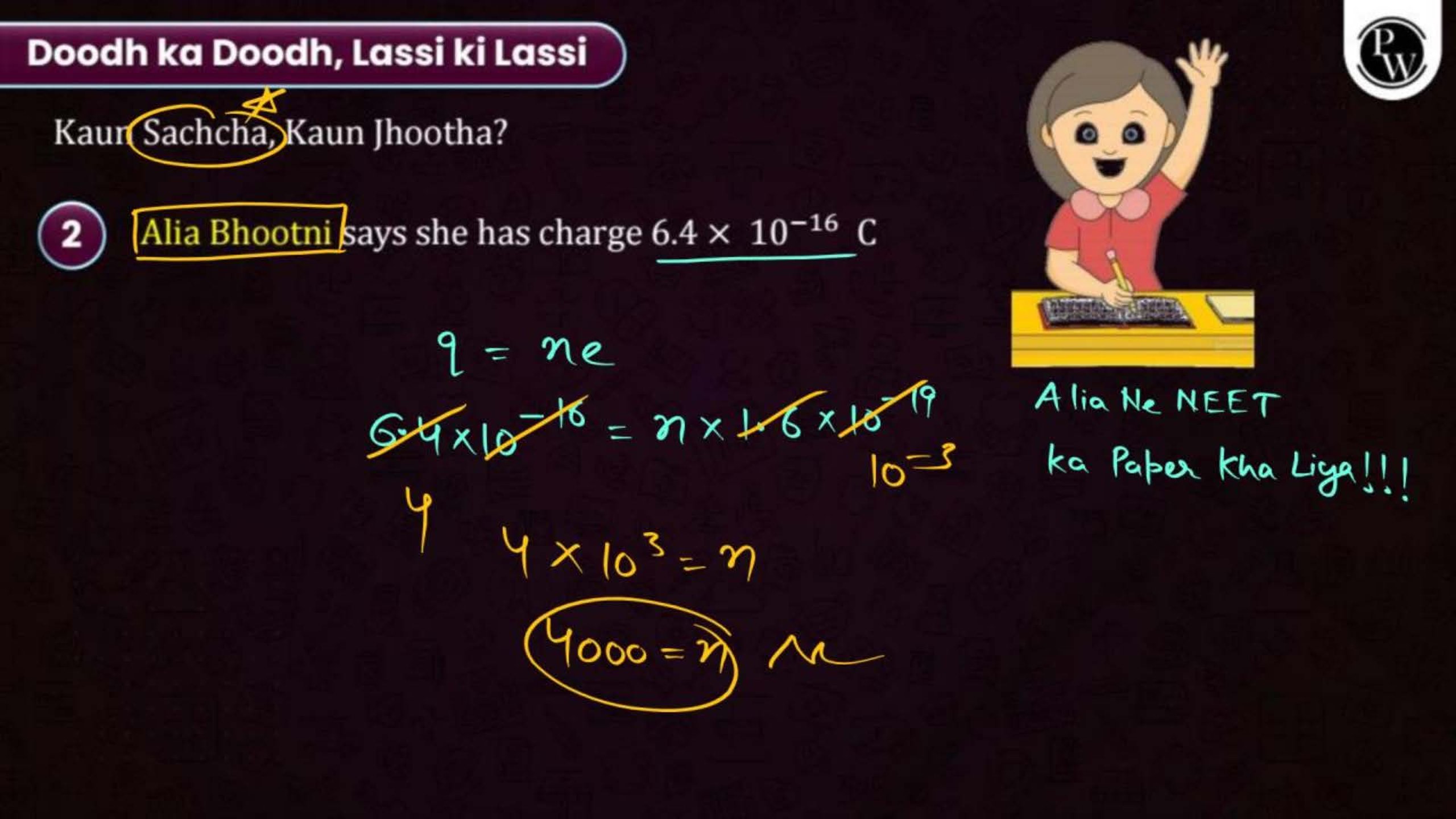
Electric Charge and Fields Notes PDF for NEET
In the Prachand Batch NEET 2025, PW provides a detailed explanation of each topic in Electric Charge and Fields. The notes cover all concepts with step-by-step explanations, diagrams, and solved examples.
Download the PDF of Electric Charge and Fields NEET Notes
To ensure a better understanding, students can also access this chapter’s video lectures on YouTube for free. These videos complement the notes, making it easier to visualize and comprehend difficult concepts. You can even download a complete chapter PDF for offline study.
[embed]https://www.youtube.com/watch?v=L8u1BzHkGNQ[/embed]What are Electric Charge and Fields?
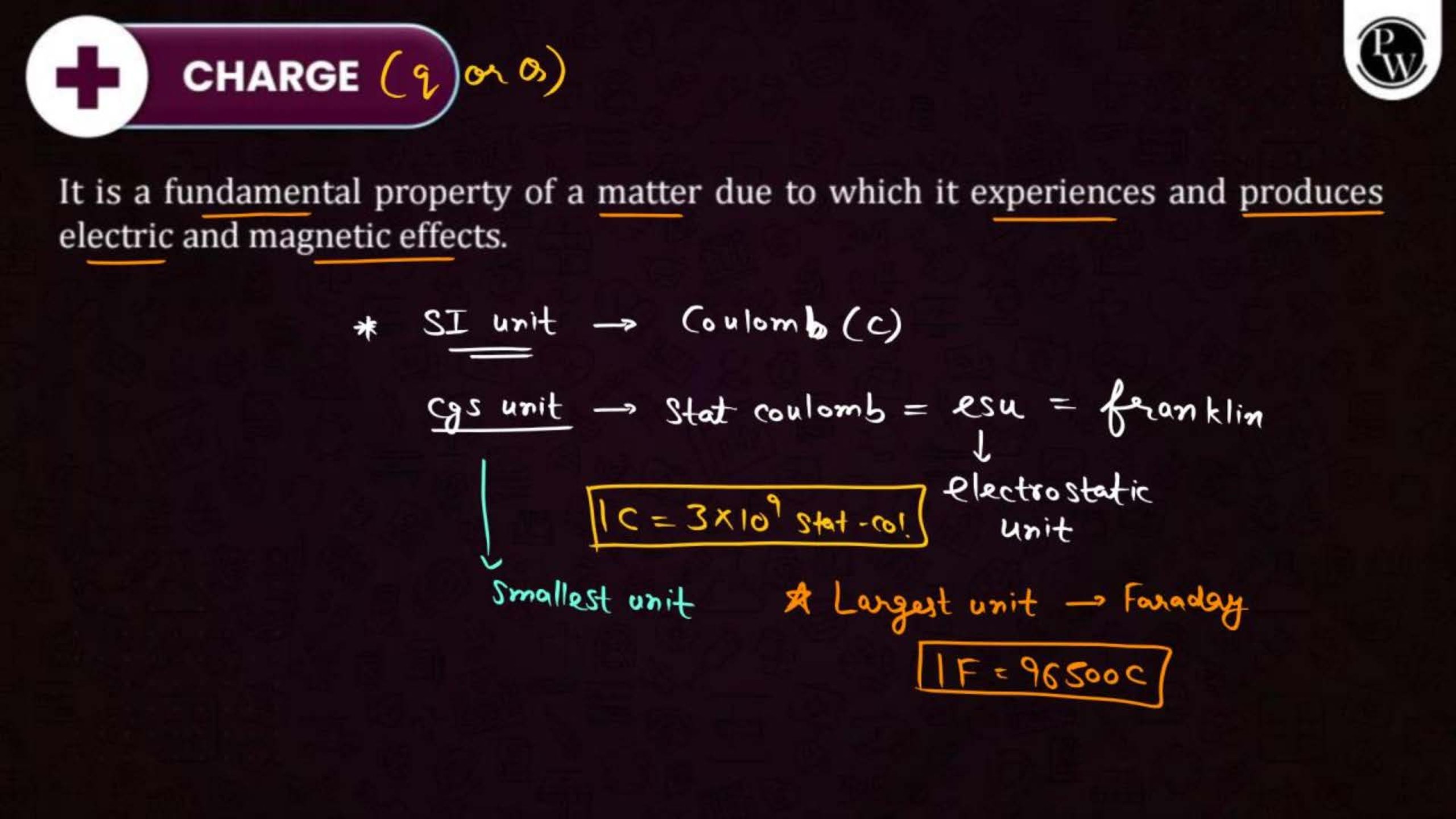
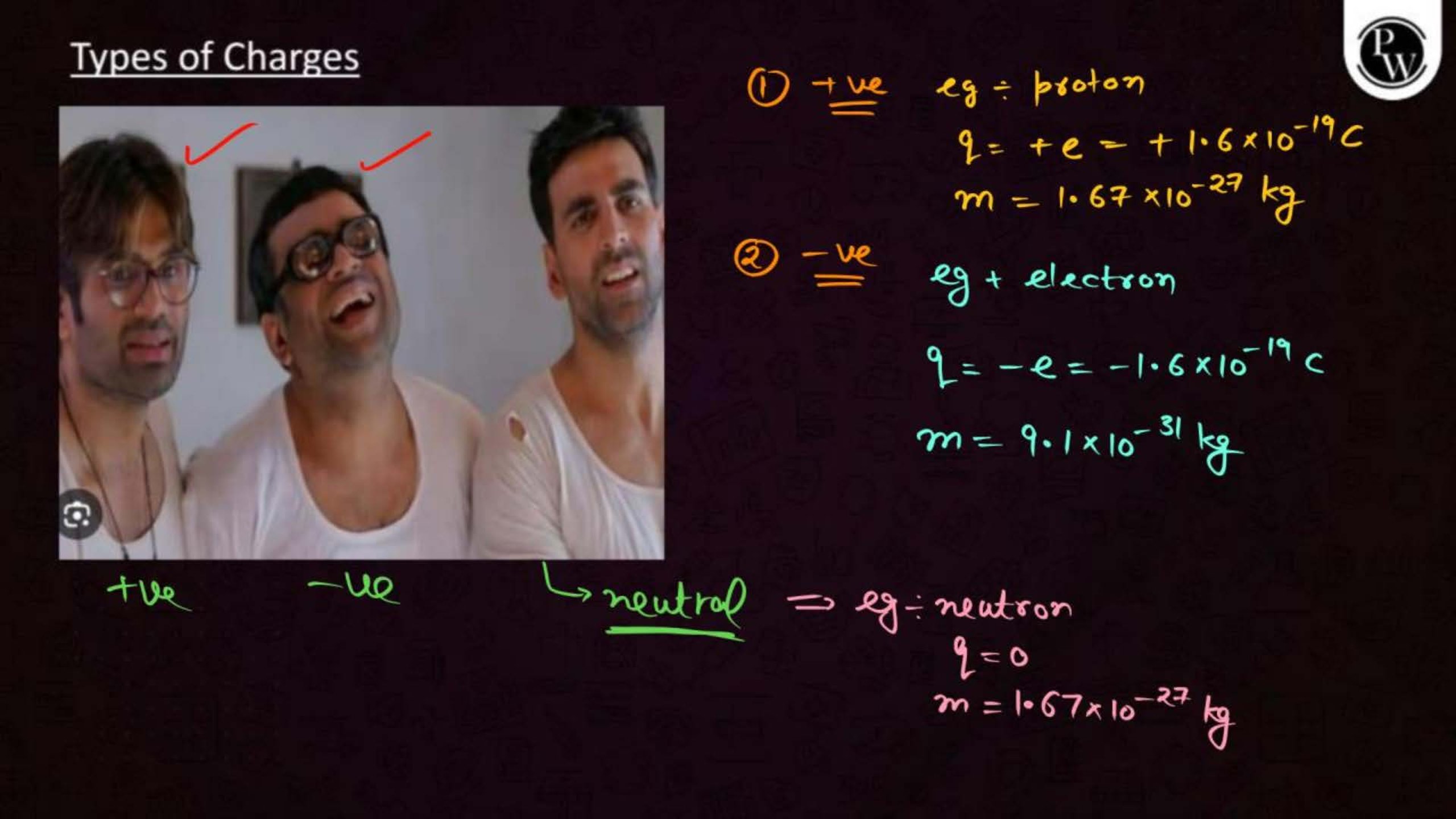
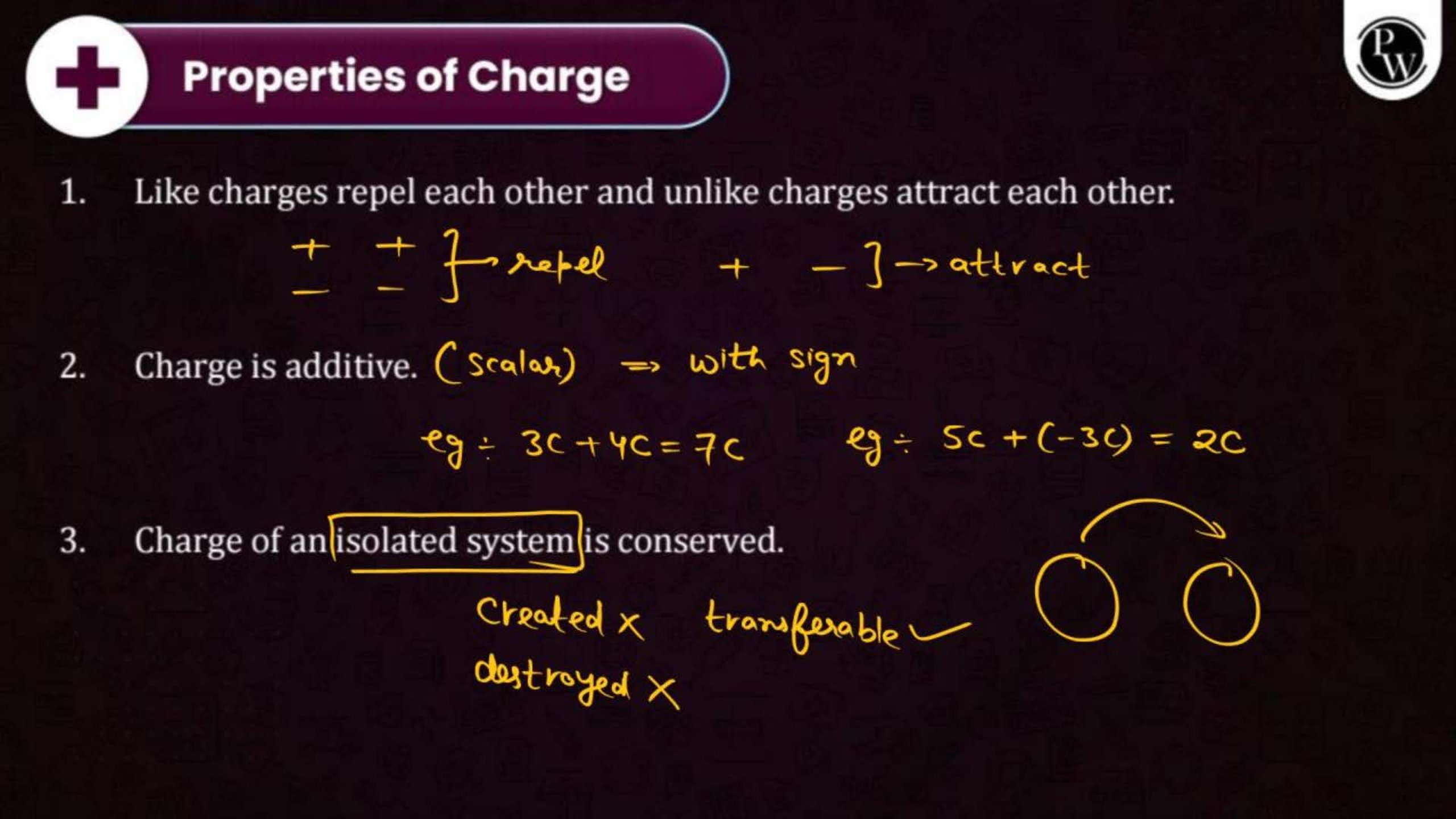
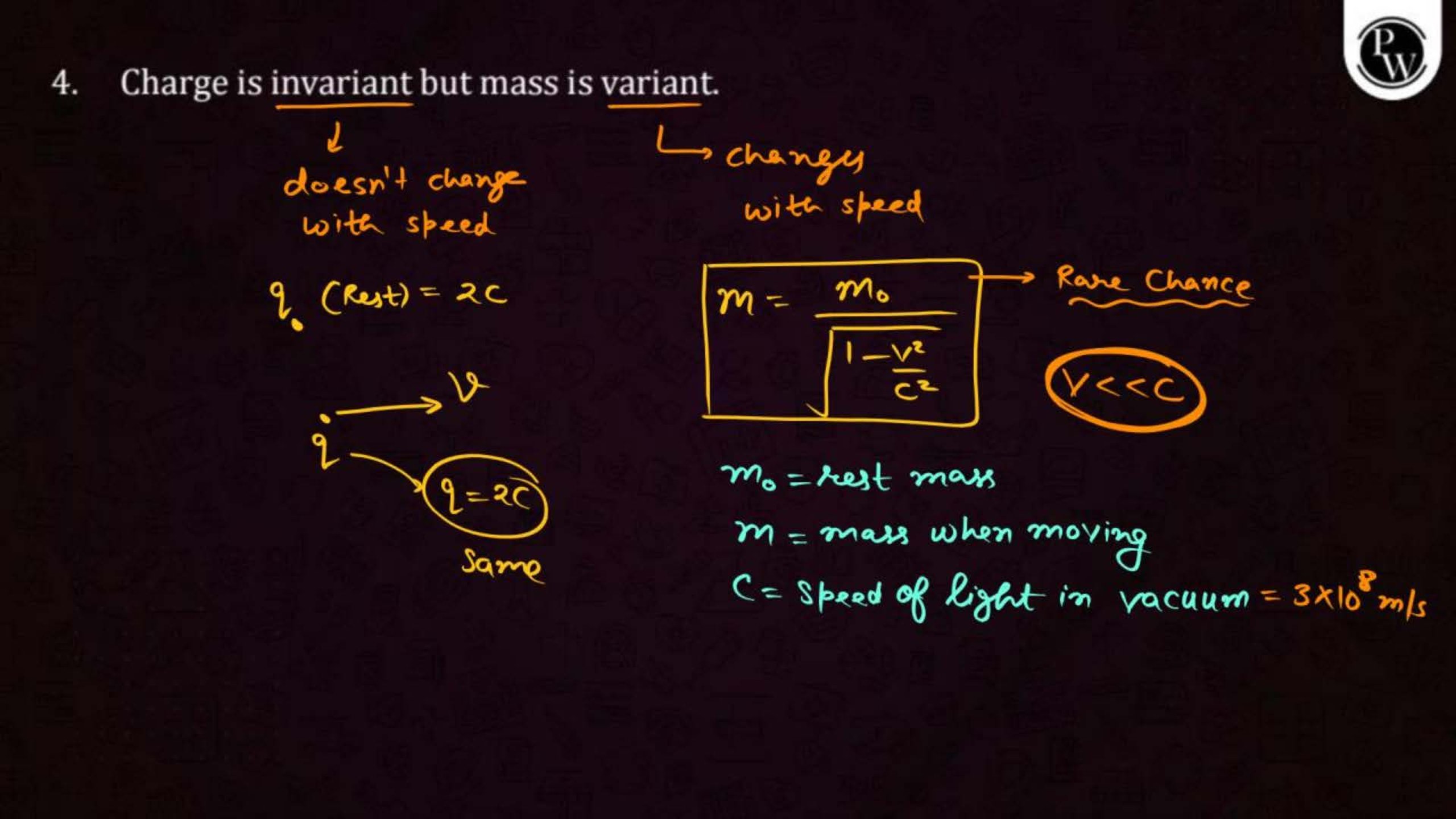
Electric charge is a property of matter that causes it to experience a force when placed in an electric field. There are two types of charges: positive and negative. Like charges repel each other, and unlike charges attract.
An electric field is the region around a charged object where its electric force can be felt by other charges. The strength of the electric field depends on the amount of charge and the distance from the charge. Electric fields are represented by field lines that show the direction and strength of the force experienced by a positive test charge.
Weightage of Electric Charge and Fields
The weightage of Electric Charge and Fields is approximately 3% in NEET 2025. This emphasizes the importance of thoroughly preparing this chapter to secure marks in Physics.
|
Weightage Following Previous Year’s Trends (2020-2024) |
||||||
| Chapter | Easy | Medium | Hard | Total | Average Q/yr | Weightage |
| Electric Charges and Fields | 0 | 4 | 2 | 6 | 1.20 | 2.38% |
| NEET Chapter Wise Weightage 2025 for Physics | |
| Topics | Weightage (%) |
| Electric Charges and Fields | 3% |
Importance of Electric Charge and Fields NEET Notes
Electric Charge and Fields NEET Notes simplify the subject by breaking down complex topics into manageable parts. They include key formulas, derivations, and practical examples that help in solving NEET-level problems. The chapter also explains real-world applications of electric charge and fields, making the concepts more relatable. With the help of these notes, students can save time during revisions and focus on understanding rather than rote memorization.
Tips for Electric Charge and Fields NEET Notes
Preparing effectively for this chapter can make solving numerical problems easier and boost your confidence in NEET. Follow these tips to master the topic with clarity and precision.
- Understand the Basics: Start by learning the basic properties of electric charge and the concept of the electric field.
- Practice Diagrams: Draw electric field lines and equipotential surfaces to visualize the concepts better.
- Learn Formulas: Memorize key formulas like Coulomb's law, electric field due to a point charge, and the principle of superposition.
- Solve Problems: Regularly practice numerical problems to strengthen your understanding and speed.
- Use Video Resources: Watch free YouTube lectures from the Prachand Batch for detailed explanations and examples.
Prepare for NEET with PW Online NEET Coaching ! Get comprehensive guidance with structured lessons, in-depth concepts, and interactive classes designed to help you succeed.
| NEET Exam Important Links | |
|---|---|
| NEET Syllabus | NEET Physics Notes |
| NEET Eligibility Criteria | NEET Exam Pattern |
| NEET Previous Year Question Papers | NEET Physics Syllabus |
Electric Charge and Fields NEET Notes
What is the relation between charge and field?
What is electric charge and field in SI units?
What is an electric field?
What are electric charges and fields formulas for NEET?