
Concept of Formal Charge
Chemical Bonding of Class 10
CONCEPT OF FORMAL CHARGE
The formal charge (F) of an atom in a polyatomic ion/molecule is the difference between the number of valence electrons in an isolated (i.e., free) atom and the number of electrons assigned to that atom, in a Lewis structure.
Formal charge (F) can be calculated by using following formula :
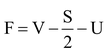
where F = Formal charge on an atom in a polyatomic ion/molecule.
V = Valence electron
S = Shared electrons (forming bonds)
U = Unshared electrons (lone pair electrons)
Let see the formal charge in the following molecule
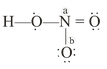
Formal charge on N atom
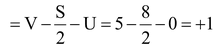
Formal charge on O atom
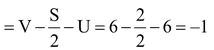
On this basis lewis structure of HNO3 may also be written as shown below :
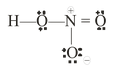
It should be noted that formal charges do not indicate real charge separation within the molecule, but indication of the formal charges on the atoms in the Lewis structure only helps in keeping track of the valence electrons in the molecule.
