Hydrogen Bonding
Chemical Bonding of Class 10
Hydrogen bond is a weak bond formed between a H atom and highly electronegative atom like O, N or F either of the same molecule or of a different molecule but to which it is not directly attached. Moreover the H atom itself should be attached to a highly electronegative atom like O, N or F.
Example: Consider the hydrogen fluorine bond in hydrogen fluoride. This bond in a polar covalent bond is which fluorine is strong electronegative element. As a result the fluorine acquires a partial negative charge and hydrogen acquires a partial positive charge.
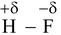
(Hydrogen Flouride)
The lone pair on the fluorine atom in another molecule of hydrogen fluoride will attract the positive charge on hydrogen in a molecule of hydrogen fluoride electrostatically. This bond between hydrogen and fluorine of different molecules is known as hydrogen bond. This new type of linkage is represented by dotted lines
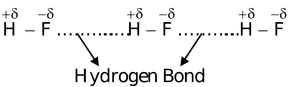
Conditions For Hydrogen Bonding
- The molecule must contain a highly electronegative atom linked to hydrogen atom.
- The size of electronegative atom should be small these condition are met in only by F, O and N atoms.
Although Cl has the same electronegativity as nitrogen, it does not form effective hydrogen bond. This is because of its larger size than that of N with the result its electrostatic attractions are weak
Types of Hydrogen Bonding
Generally the hydrogen bonds are classified into two types.
Intermolecular Hydrogen Bonding
In such types of hydrogen bonding the two or more than two molecules of same or different compounds combine together to give a polymeric aggregate.
For example,
(i) 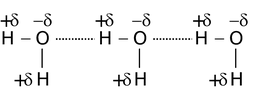
(ii) 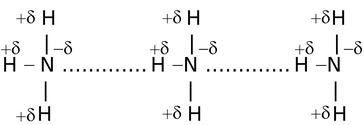
(iii) 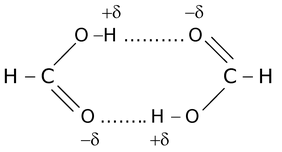
(iv) 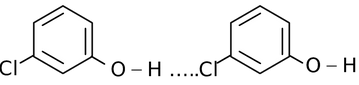
Intramolecular Hydrogen Bonding
In this type, hydrogen bonding occurs within two atoms of the same molecule. This type of hydrogen bonding is commonly known as chelation and frequently occurs in organic compounds.
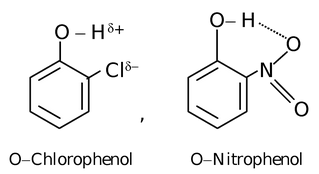
Hydrogen bonding has got a very pronounced effect on certain properties of the molecules.
State of the Substance
H2O exists in liquid state whereas H2S in gaseous state because hydrogen bonding exist in water and no H-bonding exists in H2S.
Solubility
The organic compounds like alkane, alkenes and alkynes are insoluble in water due to absence of H-bonding whereas alcohols, organic acids, amines are soluble in water due to H-bonding.
Boiling Point
High boiling and melting points of NH3,H2O and HI in comparison to hydrides of other elements of V, VI and VII groups to which N, O and F belong respectively are due to hydrogen bonding.









