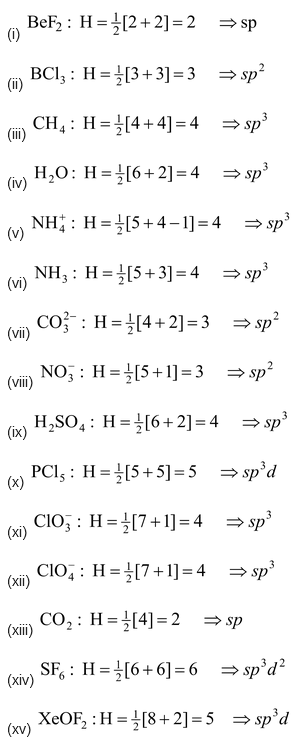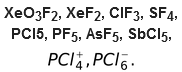Modern Theories Of Bonding
Chemical Bonding of Class 10
To explain the formation of covalent bond, following two theories based on quantum mechanics have been proposed:
- Valence bond theory
- Molecular orbit theory
Valence bond theory: Was presented by Heitler and London, in 1927 and extended by Pauling and Slater, in 1931. According to this theory, a covalent bond is formed between two atoms by partial overlap of their half-filled atomic orbitals containing electrons with opposite spin.
Characteristic of valence bond theory
- For the formation of a covalent bond, a half – filled atomic orbital of one atom overlaps with the half – filled atomic orbitals of another atom. These atomic orbitals belong to the outermost shell of the atoms.
- A covalent bond is formed by the overlapping of atomic orbitals having electrons with opposite spins.
- The atomic orbitals containing paired electrons do not participate in the processes of overlapping. These electrons are called non – bonding electrons.
- Due to the directional nature of most of the orbitals, overlapping is possible only when orbitals are properly oriented.
Hybridization
The VBT can explain satisfactorily the formation of various molecules but it fails to account the geometry and shapes of various molecules. To explain these, the concept of hybridization is necessary. This is a hypothetical concept and has been introduced by Pauling and Slater. According to this concept, any number of valance atomic orbitals of an atom which differ is energy slightly may mix with each other to form new orbitals called hybrid orbitals. The process of mixing is known as hybridisation.
Rules of hybridisation
- Orbitals having nearly same energy belonging to same atom can take part e.g 2s orbital can mix-up with 2p, but cann’t mix-up with 3p orbitals.
- Number of hybrid orbitals are equal to the total number atomic orbitals take part.
- Most of the hybrid orbitals are similar in shape but they are not necessarily identical in shape.
- It is the orbitals that undergo hybridisation and not the electron.
- The electron waves in hybrid orbitals repel each other and this tend to make them far apart.
- Hybrid orbitals form only sigma bonds.
- Depending on the number and nature of hybrid orbitals different geometry will be achieved by the molecule due to different orientation of hybrid orbitals in spare. If the central atom in the molecule does not contain any lone pair, it will show regular geometry.
If the central atom is surrounded by one or more orbitals containing lone pairs of electrons in the valency shell, the geometry of the molecule is distorted to some extent due to lone pair bond pair repulsion.
Types of hybridization
(i) sp (ii) sp2 (iii) sp3 (iv) sp3d1 (v) sp3d2 (vi) sp3d2
(i) sp Hybridization
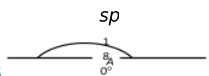
It involves the combination of one s and one p−atomic orbital and gives two equivalent orbitals known as sp−hybrid orbitals.
s + p →sp + sp
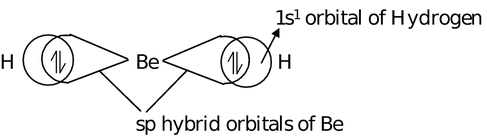
The sp hybrid orbitals lie diagonally opposite to each other, i.e. in a straight line subtending an angle of 180° between them. Each sp−hybrid orbital has 50% s−character and 50% p−character. The effective size of sp−hybrid orbitals is smaller than sp2 and sp3 hybrid orbitals. This type of hybridization takes place in the formation of alkynes, CO2, BeF2, BeCl2, BeH2, HgCl2 etc. Let us consider the formation of BeH2.
Electronic configuration of Be is = 1s2, 2s2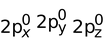 (Ground state)
(Ground state)
= 1s2, 2s1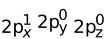 (Exited state)
(Exited state)
(One electron promoted from 2s to 2px)
Now
s + p = sp + sp
and
(ii) sp2 Hybridization
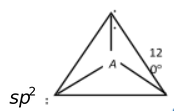
When one s and two p orbitals, of an atom are mixed to give three new hybrid orbitals, the process is called sp2−hybridization. The three equilant new orbitals lie in one plane and are directed towards the three corners of an equilateral triangle. The angle between sp2−hybrid orbitals is 120° to have minimum repulsion. The s character in sp2 hybrid orbital is 33% and p character is 67%. This type of hybridization takes place in the formation of alkenes, BF3, BCl3, 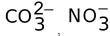 etc. Let us discuss the hybridization of BF3.
etc. Let us discuss the hybridization of BF3.
Electronic configuration of B = 1s2, 2s2 2p1 (Ground state)
= 1s2, 2s1 (Exited state)
(Exited state)
(In the exited state one s electron promoted to 2py
atomic orbital)
Now
s + p + p  sp2 + sp2 + sp2
sp2 + sp2 + sp2
each of these sp2 hybrid orbitals of boron overlaps with the unpaired electron in 2pz orbital of each three fluorine atoms. The shape of BF3 molecule is plane triangular with a bond angle 120°.
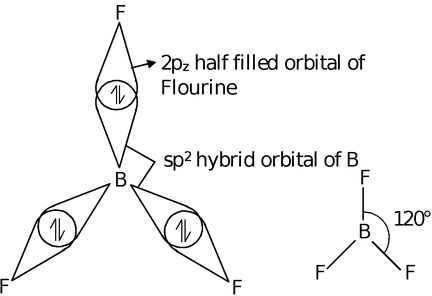
The vacant 2pz atomic orbital of boron is present on boron in BF3 molecule in a plane perpendicular to the plane of molecule. Boron can accept one lone pair in the atomic orbital.
(iii) sp3 Hybridization
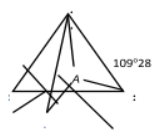
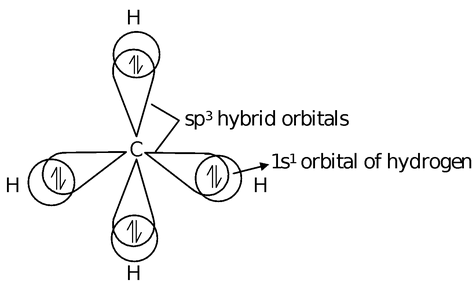
This hybridization involves the inter mixing of one s− and three p− atomic orbitals to give four sp3 hybrid orbitals. These sp3 orbitals are directed towards the four corners of a regular tetrahedron hence the name tetrahedral hybridization. The hybrid orbitals subtend an angle of 109°, 28' for minimum repulsion. The 's' character in sp3 hybrid orbital is 25% while 'p' character is 75%.
s + p + p + p = sp3 + sp3 + sp3 + sp3
This type of hybridization takes place in carbon in saturated organic compounds (alkanes) and in NH3, SiF4, SiCl4, H2O, SO--4 etc. Let us discuss the formation of methane.
Formation of CH4: Electronic configuration of C
= 1s2, 2s2 2p2 (Ground state)
= 1s2, 2s1 2p3 (Excited state)
s + p + p + p = sp3 + sp3 + sp3 + sp3
These sp3 hybrid orbitals of carbon overlaps with one 's' orbital of hydrogen to form CH4.
|
Type of Hybridisation |
Character |
Shape of the molecule or ion |
Example |
|
|
s-character = 50%, p-character = 50%. |
Linear |
|
|
|
s-character = 33.33%, p-character = 66.67% |
Triangular planar |
|
|
sp3 |
s-character = 25%, p-character = 75% (as s-character decreases, p-character increases, bond angle decreases). |
Tetrahedral |
|
|
sp3d
|
s-character = 20%, p-character = 60%, d-character = 20% |
Trigonal bipyramidal |
|
| sp3d2 |
s-character = 16.66%, p-character = 49.98%, d-character = 33.33% |
Octahedral |
SF6, MoF6, XeF4, BrF5, XeOF4, [BiCl6]–, [PF6]–, [Co(NH3)6]3+. |
|
sp3d3
|
s-character = 14.28%, p-character = 42.86%, d-character = 42.86% |
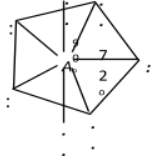
Pentagonal bipyramidal |
IF7, XeF6, [ZrF7]3–, [UF7]3–, [UO2F5]3– etc. |
Method of predicting the hybrid state of the central atom in covalent molecules or polyatomic ions
|
Type of hybridization of central atom of a simple molecule or ion can be determined with the help of the value of ‘H’ which is equal to:
|
Examples: