Shape Of Molecules
Chemical Bonding of Class 10
Once you know the type of hybridization, shape of molecule can be predicted by the following table. Shape of molecule is not always equal to shape of hybridization, it depends on type of atom attached to the central atom.
| Type of molecule | No. of bond pairs of electrons | No. of lone pairs of electrons | Hybridization | Bond angle | Geometry Of Molecule | Shape of molecule |
|---|---|---|---|---|---|---|
| AX3 | 2 | 1 | sp2 | • 120° | Trigonal planar | V-shape Bent |
| AX4 | 2 | 2 | sp3 | • 109°28′ | Tetrahedral | Angular V-shape |
| AX4 | 3 | 1 | sp3 | • 109°28′ | Tetrahedral | Pyramidal |
| AX5 | 4 | 1 | sp3d | • 109°28′ | Trigonal bipyramidal | Irregular tetrahedron |
| AX5 | 3 | 2 | sp3d | 90° | Trigonal bipyramidal | T-shape |
| AX5 | 2 | 3 | sp3d | 180° | Trigonal bipyramidal | Linear |
| AX6 | 5 | 1 | sp3d2 | • 90° | Octahedral | Square pyramidal |
| AX6 | 4 | 1 | sp3d2 | –– | Octahedral | Square planar |
| AX7 | 6 | 1 | sp3d2 | –– | Pentagonal pyramidal | Distorted octahedral |
Dipole Moment :
The percentage of polar character in a covalent bond is compared in terms of dipole moment (μ) It is defined as the product of magnitude of charge developed on any of the atom and the distance between the atoms. In general, the molecules having μ = 0 are called non polar molecules and molecules having μ > 0 are called polar molecules.
- Dipole moment is a vector quantity.
- Non polar diatomic molecules have a zero dipole moment while diatomic molecules formed by atoms of different electronegativity possess dipole mements.
- Dipolemoment of polyatomic molecules is taken as the resultant of all the bonds. For example
(i) Molecules with regular geometry have a zero dipole moment, while
(ii) Molecules with irregular geometry (having b.p. and I.P) possess a definite dipole moment.
Dipole moment of some common substance
| Substance | Dipole moment (D) | Substance | Dipole moment (D) |
|---|---|---|---|
| HF H2O SO2 NH3 NF3 | 1.91 1.84 1.60 1.46 0.24 | CH3Cl HCl H2S HBr HI | 1.86 1.03 1.10 0.78 0.38 |
For example,
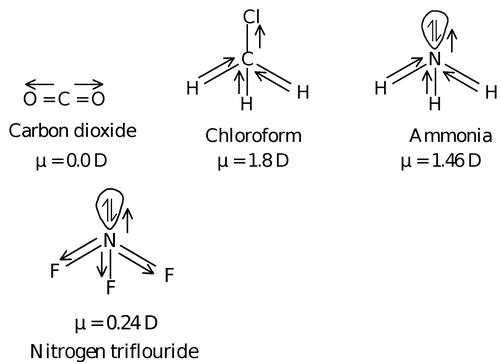
Since dipole moments of the lone pair and bond electron pairs are in the opposite direction therefore dipole moment of NF3 is very low.
Dipole moment in organic compounds
(i) In geometrical isomerism, trans isomer has either zero dipole moment or very less depending on the structure of alkene.
Example
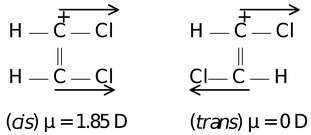
cis−1,2−dichloroethene trans−1,2−dichloroethene
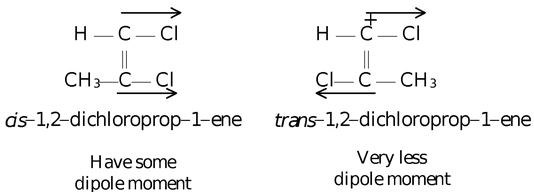

The dipole moment of trans−1,2−dichloro prop−1−ene is very less. Because magnitude of upper vector is not equal to the magnitude of lower vector









