
Ortho-Para-Directing Groups
Electrophilic Aromatic Substitution of Class 12
Ortho-Para-Directing Groups
Except for the alkyl and phenyl substituents, all of the ortho-para directing groups in are of the following general type :
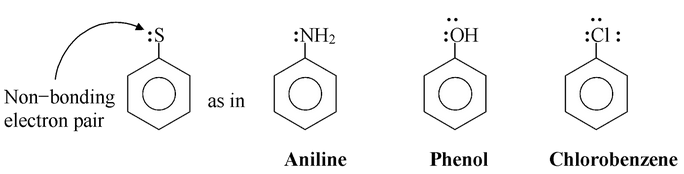
All of these ortho-para directors have at least one pair of nonbonding electrons on the atom adjacent to the benzene ring.
This structural feature - an unshared electron pair on the atom adjacent to the ring – determines the orientation and influences reactivity in electrophilic substitution reactions.
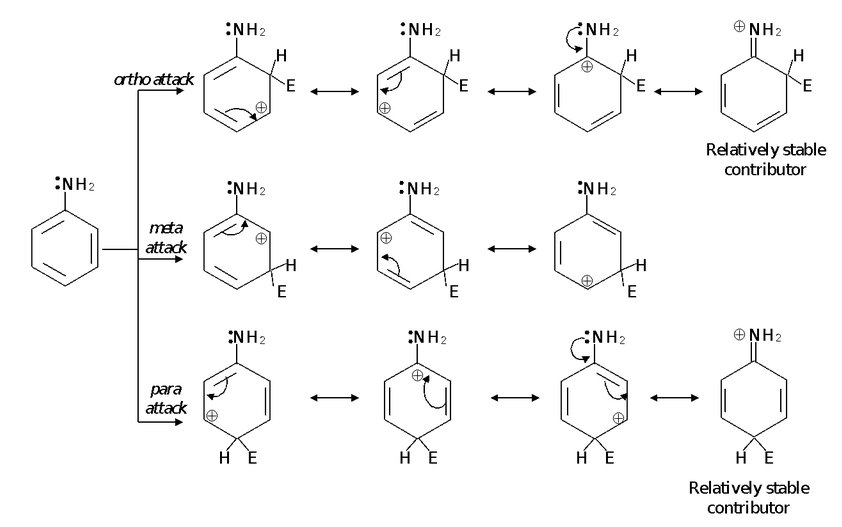
We see that four reasonable resonance structures can be written for the arenium ions resulting from ortho and para attack, whereas only three can be written for the arenium ion that results from meta attack. This, in itself, suggests that the ortho- and para-substituted arenium ions should be more stable. Of greater importance, however, are the relatively stable structures that contribute to the hybrid for the ortho- and para-substituted arenium ions. In these structures, nonbonding pairs of electron from nitrogen form an extra bond to the carbon of the ring. This extra bond and the fact that every atom in each of these structures has a complete outer octet of electrons – makes these structures the most stable of all of the contributors. Because these structures are unusually stable, they make a large – and stabilizing– contribution to the hybrid. This means, of course, that the ortho- and para-substituted arenium ions themselves are considerably more stable than the arenium ion, that results from the meta attack.
In case of halogens inductive effect predominates over the mesomeric effect, due to which they act as ring deactivators instead of ring activators.
