
Ortho−Para Direction And Reactivity Of Alkylbenzenes
Electrophilic Aromatic Substitution of Class 12
Alkyl groups are ortho-para directors. We can also account for this property of alkyl groups on the basis of their ability to release electrons - an effect that is particularly important when the alkyl group is attached directly to a carbon that bears a positive charge.
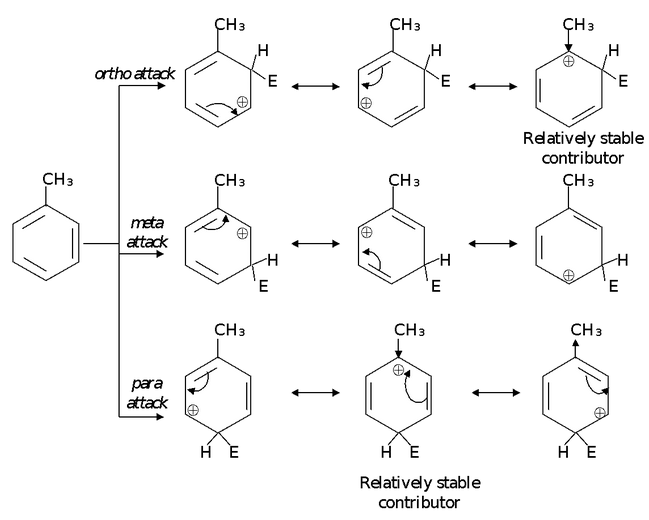
In ortho attack and para attack we find that we can write resonance structures in which the methyl group is directly attached to a positively charged carbon of the ring. These structures are more stable relative to any of the others because in them the stabilizing influence of the methyl group (by electron release) is most effective. These structures, therefore, make a large (stabilizing) contribution to the overall hybrid for ortho - and para - substituted arenium ions.
