
Theory Of Substituent Effects On Electrophilic Aromatic Substitution
Electrophilic Aromatic Substitution of Class 12
Theory Of Substituent Effects On Electrophilic Aromatic Substitution
Reactivity: The Effect of Electron-Releasing and Electron-Withdrawing Groups
We have seen that certain groups activate the benzene ring toward electrophilic substitution, while other groups deactivate the ring. When we say that a group activates the ring, what we mean, of course, is that the group increases the relative rate of the reaction. We mean that an aromatic compound with an activating group reacts faster in electrophilic substitutions than benzene. When we say that a group deactivates the ring, we mean that an aromatic compound with a deactivating group reacts slower than benzene.
We find that the relative rates of the reactions depend on whether S withdraws or releases electrons. If S is an electron-releasing group (relative to hydrogen), the reaction occurs faster than the corresponding reaction of benzene. If S is an electron-withdrawing group, the reaction occurs slower than that of benzene.
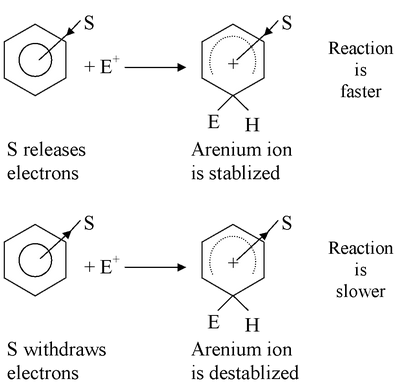
It appears, then, that the substituent (S) must affect the stability of the transition state relative to that of the reactants. Electron-releasing groups apparently make the transition state more stable, while electron-withdrawing groups make it less stable. This is fairly reasonable, because the transition state resembles the arenium ion, and the arenium ion is a delocalized carbocation.
