
Some Important Electrophilic Aromatic Substitution Reactions
Electrophilic Aromatic Substitution of Class 12
(i) Halogenation
Benzene does not react with bromine or chlorine unless a Lewis acid is present in the mixture, (as a consequence, benzene does not decolorize a solution of bromine in carbon tetrachloride). When Lewis acids are present, however, benzene reacts readily with bromine or chlorine, and the reactions give bromobenzene and chlorobenzene in good yields.

The Lewis acids most commonly used to effect chlorination and bromination reactions are FeCl3, FeBr3, and AlCl3 all in the anhydrous form. Ferric chloride and ferric bromide are usually generated in the reaction mixture by adding iron to it. The iron then reacts with halogen to produce the ferric halide.
2Fe + 3X2 → 2FeX3
The mechanism for aromatic bromination is as follows :
Step−1: Bromine combines with FeBr3 to form a complex that dissociates to form a positive bromine ion and 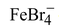 .
.
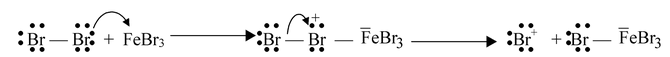
Step−2: The positive bromine ion attacks benzene to form an arenium ion.
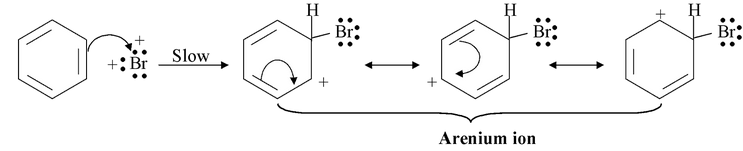
Step−3: The arenium ion loses a proton to become bromobenzene.
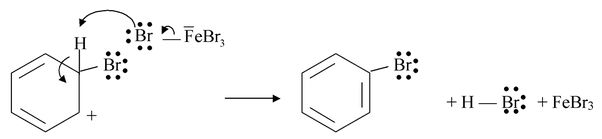
Iodine, on the other hand, is so unreactive that a special technique has to be used to effect direct iodination. The reaction has to be carried out in the presence of an oxidizing agent such as nitric acid or iodic acid.
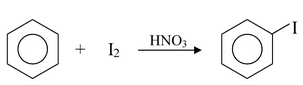
(ii) Nitration
Benzene reacts slowly with hot concentrated nitric acid to yield nitrobenzene. The reaction is much faster if it is carried out by heating benzene with a mixture of concentrated nitric acid and concentrated sulfuric acid.
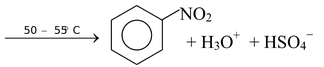
☞ Concentrated sulphuric acid increases the rate of the reaction by increasing the concentration of the electrophile− the nitronium ion.
Step−1 Nitric acid acts as a base and accepts a proton from the stronger acid, sulfuric acid.
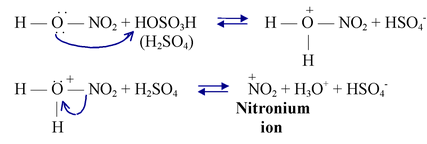
The protonated nitric acid dissociates and produces a nitronium ion.

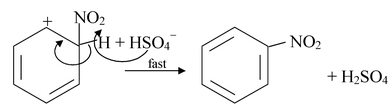
The nitronium ion reacts with benzene by attacking the π cloud and forming an arenium ion.
The arenium ion then transfers a proton to some base in the mixture such as HSO4- and becomes nitrobenzene.
Step−3:
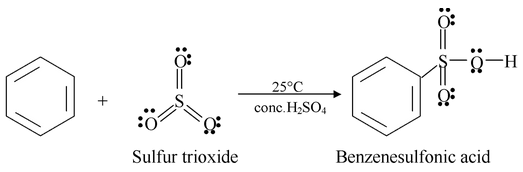
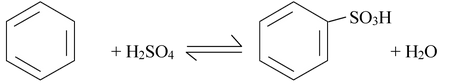
(iii) Sulfonation
Benzene reacts with fuming sulfuric acid at room temperature to produce benzenesulfonic acid. Fuming sulfuric acid is sulfuric acid that contains added sulfur trioxide (SO3). Sulfonation also takes place in concentrated sulfuric acid alone, but more slowly.
Step −1: In concentrated sulphuric acid, sulfur trioxide is produced in the following equilibrium in which H2SO4 acts both as an acid and a base.
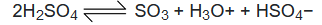
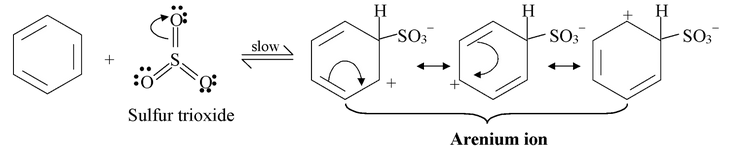
Step−2
Step−3
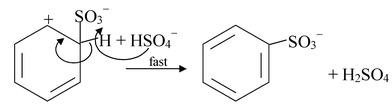
Step−4
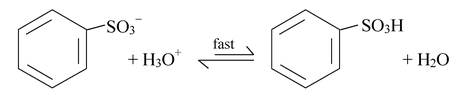
All the steps in sulphonation are in equilibrium, including step 1 in which sulfur trioxide is formed from sulfuric acid. This means that the overall reaction is an equilibrium as well. In concentrated sulfuric acid, the overall equilibrium is the sum of steps 1 to 4.
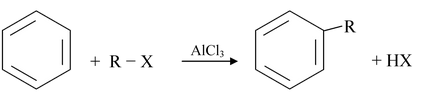
(iv) Friedel−Crafts Alkylation
A general equation for a Friedel - Crafts alkylation reaction is as follows:
Mechanism:−
Step 1 
Isopropyl chloride Carbocation
Step 2 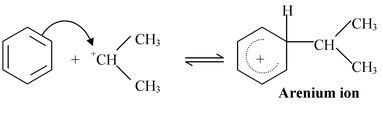
Step 3 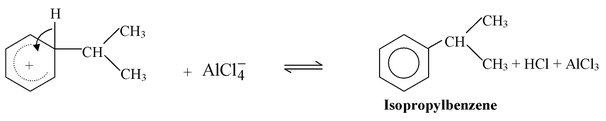
Examples:
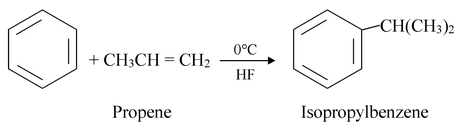
A mixture of an alcohol and an acid may also be used.
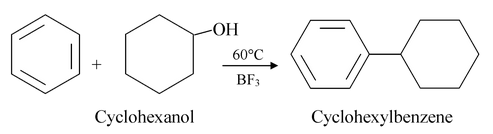
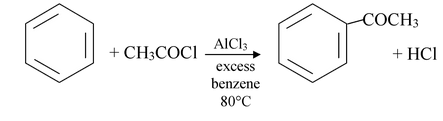
(v) Friedel Crafts acylation
The RCO− group is called an acyl group, and a reaction whereby an acyl group is introduced into a compound is called acylation reaction.
The Friedel - Crafts acylation reaction is an effective means of introducing an acyl group into an aromatic ring. The reaction is often carried out by treating the aromatic compound with an acyl halide. Unless the aromatic compound is one that is highly reactive, the reaction requires the addition of atleast one equivalent of a Lewis acid (such as AlCl3) as well. The product of the reaction is an aryl ketone.
Acetyl Acetophenone
chloride (methyl phenyl ketone)
Acyl chlorides, also called acid chlorides, are easily prepared by treating carboxylic acids with thionyl chloride (SOCl2) or phosphorus pentachloride (PCl5).
CH3CO2H + SOCl2 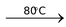 CH3COCl + SO2 + HCl
CH3COCl + SO2 + HCl
Acetic Thionyl Acetyl
acid chloride chloride
PhCO2H + PCl5 → PhCOCl + POCl3 + HCl
Benzoic Phosphorus Benzoyl
acid pentachloride chloride
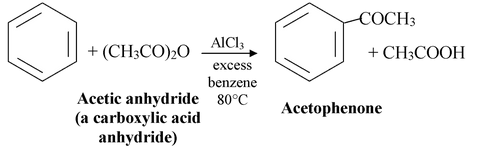
Friedel - Crafts acylations can also be carried out using carboxylic acid anhydrides. For example :
Mechanism:
In most Friedel - Crafts acylations the electrophile appears to be an acylium ion formed from an acyl halide in the following way :
Step−1
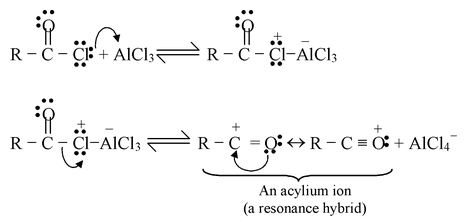
Step −2
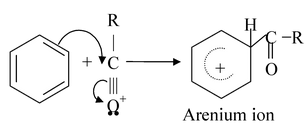
Step−3
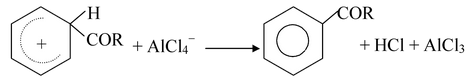
Step−4
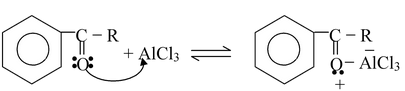
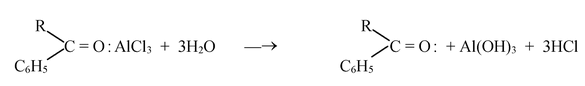
☞ In the last step aluminum chloride (a Lewis acid) forms a complex with the ketone (a Lewis base). After the reaction is over, treating the complex with water liberates the ketone.
Limitations of Friedel −Crafts reaction
1. When the carbocation formed from an alkyl halide, alkene, or alcohol can rearrange to a more stable carbocation, it usually does so and the major product obtained from the reaction is usually the one from the more stable carbocation.
When benzene is alkylated with butyl bromide, e.g. some of the developing butyl cations rearrange by a hydride shift –– some developing 1o carbocations (see following reactions) become more stable 2o carbocations. Then benzene reacts with both kinds of carbocations to form both n−butylbenzene and sec-butyl-benzene.
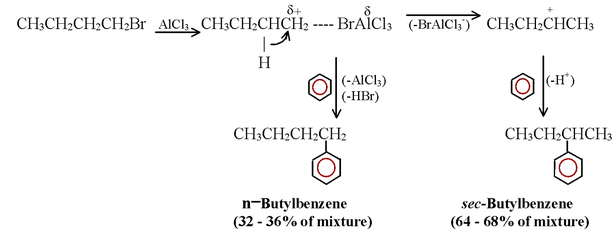
2. Friedel - Crafts reactions do not occur when powerful electron-withdrawing groups are present on the aromatic ring or when the ring bears an –NH2, –NHR, or –NR2 group. This applies to alkylations and acylations.
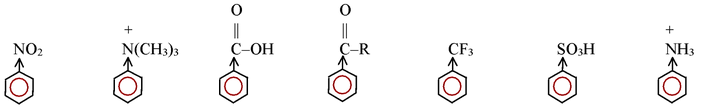
We shall learn that groups present on an aromatic ring can have large effect on the reactivity of the ring towards electrophilic aromatic substitution.
Electron - withdrawing groups make the ring less reactive by making it electron deficient. Any substituent more electron withdrawing (or deactivating) than a halogen, that is, any meta-directing group, makes an aromatic ring too electron deficient to undergo a Friedel - Crafts reaction. The amino groups, –NH2, –NHR, and –NR2 are changed into powerful electron - withdrawing groups by the Lewis acids used to catalyze Friedel - Crafts reactions. For example :
3. Aryl and vinylic halides cannot be used as the halide component because they do not form carbocations readily.
