Alkenes
Hydrocarbon of Class 12
Nomenclature
The alkenes are unsaturated hydrocarbons that contain one double bond. They have the general formula CnH2n and the double bond is known as olefinic bond or ethylenic bond.
e.g.
CH2 = CH2 Ethene
CH3 CH = CH2 Propene
CH3CH = CHCH3 But – 2 – ene
METHODS OF PREPARATION
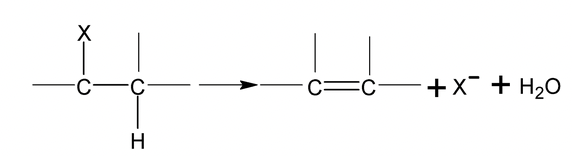
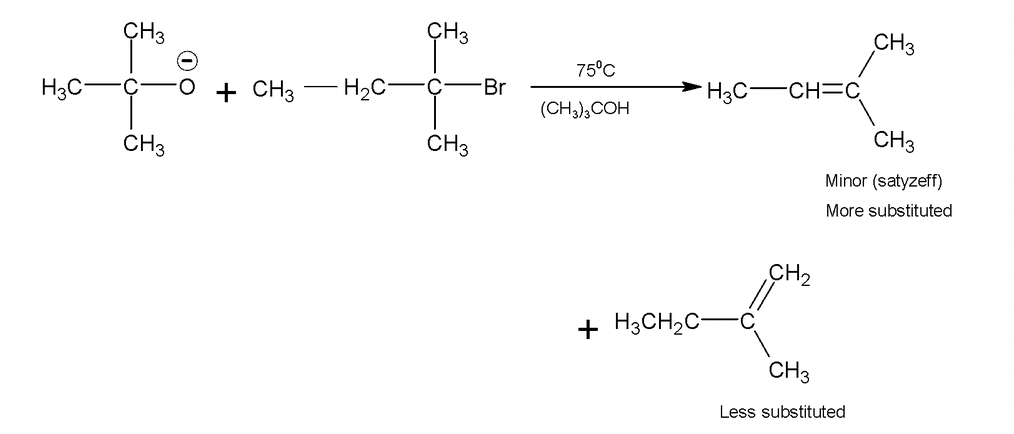
1. Dehydrohalgoenation
It is possible to form alkenes by base – induced elimination from alkyl halides.
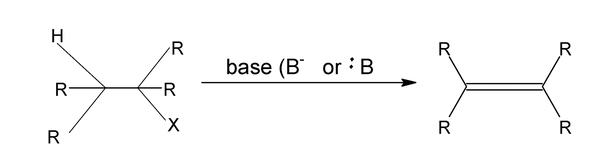
Alcoholic KOH converts alkyl halide into alkene by a reaction called dehydrohalogenation which involves removal of the halogen atom together with a hydrogen atom from a carbon adjacent to the one having the halogen.
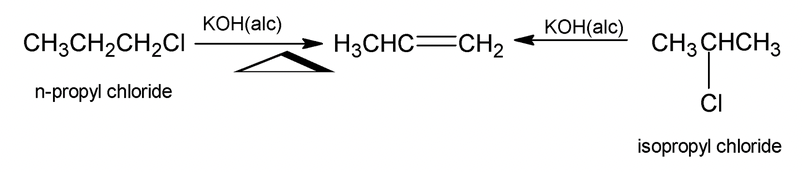
.png)
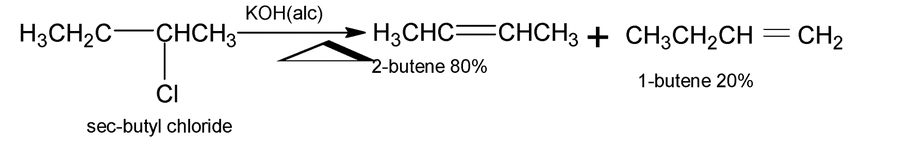
Note:
(i) Ease of dehydrohalogenation of alkyl halides is in order 30 > 20 > 10.
(ii) Ease of formation of alkenes is in order
R2C = CR2 > R2C = CHR > R2C = CH2 or RCH = CHR > RCH = CH2 > CH2= CH2 and same is the stability order of alkenes.
Also
|
|
> |
|
≈ |
|
Thus by general rule: More stable the alkene, the more easily it is formed
Increasing rate of dehydrohalogenation is in the order.
RF < RCl < RBr < RI
Also greater the conjugation, greater is the stability (due to resonance) hence easier is the dehydrohalogenation.
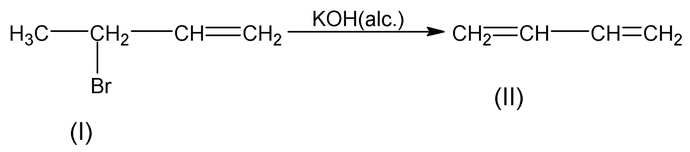
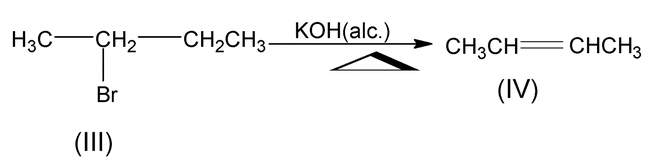
As II is more stable than IV hence dehydrohalogenation of I is easier than that of (III).
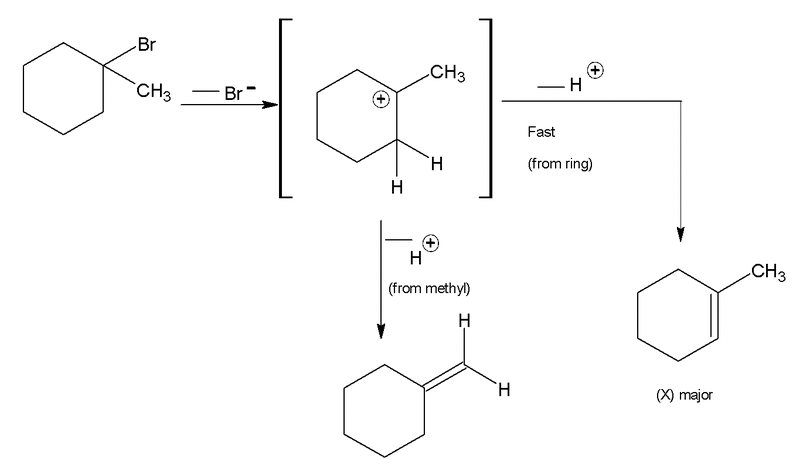
In case of elimination of HBr from 1 – bromo – 1 – methyl cyclohexane, loss of bromide provides a tertiary cation. This species is symmetrical and loss of proton from either of the adjacent methylene groups leads to the same product, 1 – methyl – 1 cyclohexene in which the double bond is in the ring (endocyclic) on the other hand loss of proton from the methyl group produces methylene cyclohexane (Y) in which the double bond is outside the ring (exocyclic). By saytzeff rule, the more highly substituted an alkene, the more stable it is hence formation of 1 – methyl – 1 – cyclohexene is favoured.
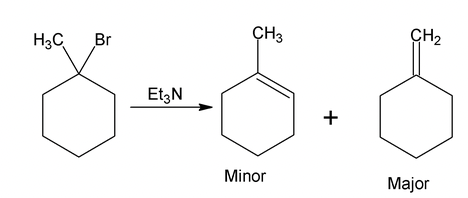
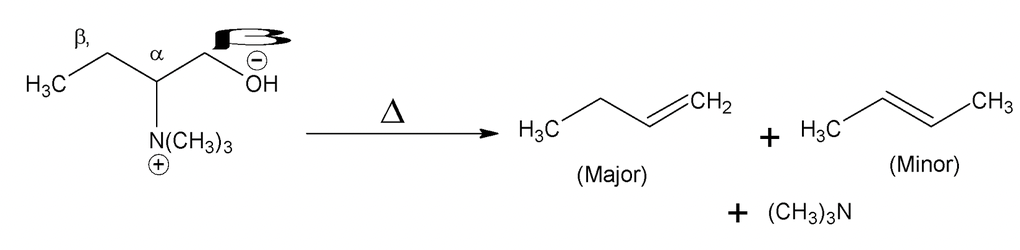
Formation of less substituted alkene in an elimination reaction is referred to as a Hofmann elimination.
Note:
Hindered base gives Hofmann product as major isomer.
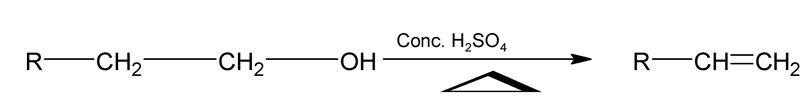
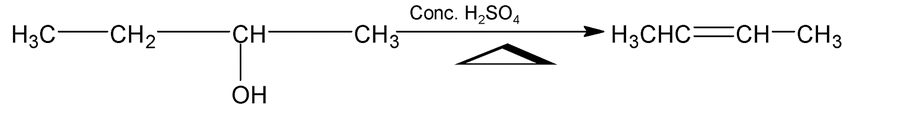
2. Dehydration of Alcohols
Alcohols undergo dehydration to give alkenes.
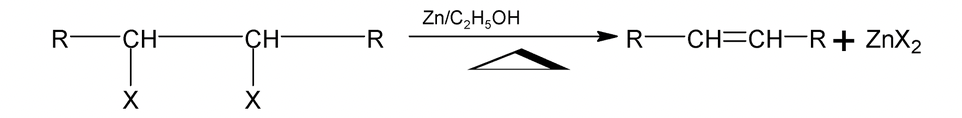
3. Dehalogenation
(i) Vicinal dihalides undergo dehalogenation in the presence of Zn, Ag or Mg.
(ii) Geminal dihalides undergo coupling reaction Via dehalogenation to give alkenes.
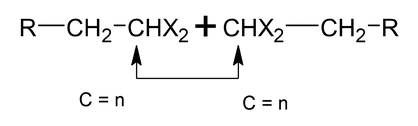
R ⎯ CH2 ⎯ CH = CH ⎯ CH2 ⎯ R
(C = 2n)
4. Thermal elimination reaction
The product formation takes place by Hofmann rule. Following compounds give thermal elimination reactions.
(i) Acetates
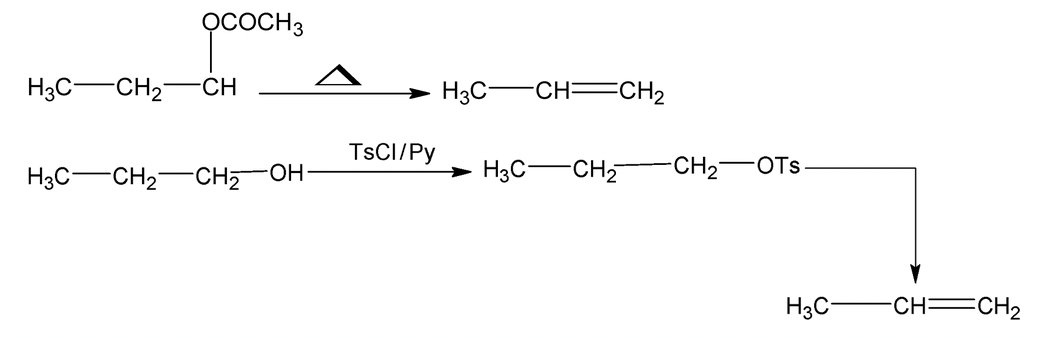
(ii) Amine oxide
Thermal elimination of amine oxides is known as copper elimination.
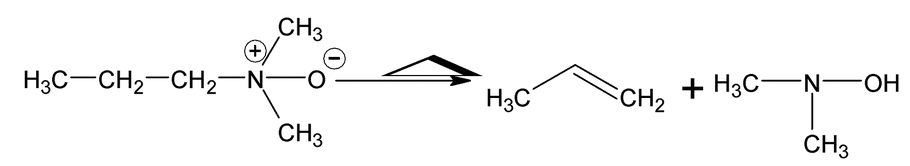
(iii) Quaternary ammonium hydroxide
Thermal elimination of this compound is known as Hofmann elimination.
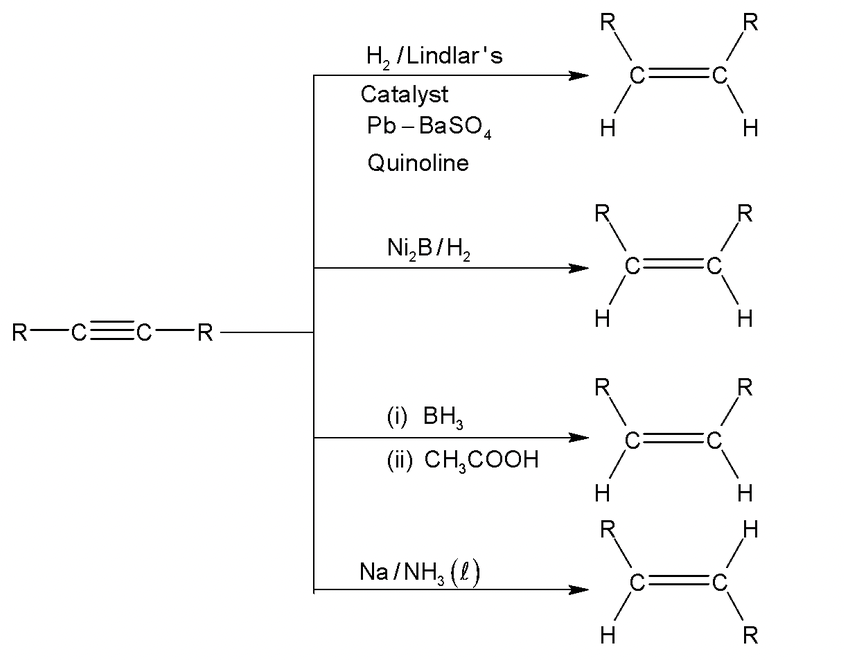
5. By partial reduction of alkynes:
Alkynes undergo partial reduction to give alkenes in the presence of catalyst
6. Wittig Reaction
Carbonyl compounds react with 10 and 20 alkyl halides in the presence of triphenyl phosphine and strong base (RLi, NaH etc) to give alkenes. This reaction is known as Wittig reaction.
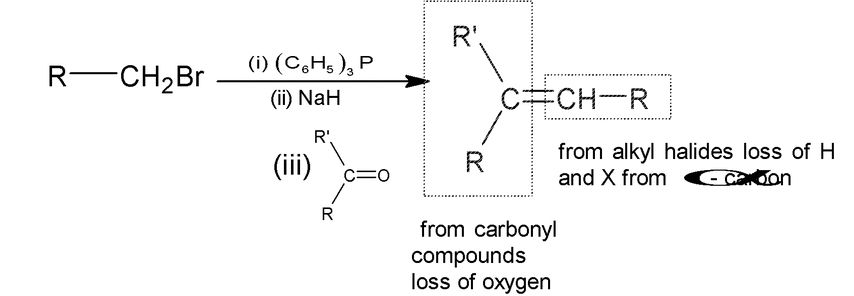
Note:
For writing product remove H and X from the α - carbon of alkyl halide and oxygen from- carbonyl carbon and join these two carbons (α - carbon and carbonyl carbon) by double bond.
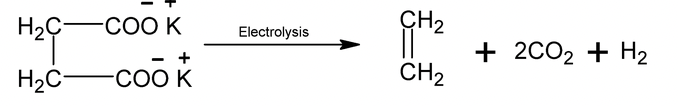
7. Kolbe hydrocarbon synthesis
Electrolysis of potassium salt of succinic acid gives alkene at the anode
PHYSICAL PROPERTIES
At room temperature alkenes differs in their physical state depending upon the number of carbon atom.
C2 ⎯ C4 : Gases
C5 ⎯ C17 : Liquids
C18 ⎯ Onwards : Solids like alkanes
CHEMICAL PROPERTIES
Alkenes show
(a) Free – radical attack (Substitution reaction)
(b) Ionic – attack (Addition reaction)
The Type of reaction depends upon experimental conditions.
Some reactions of alkenes are given below
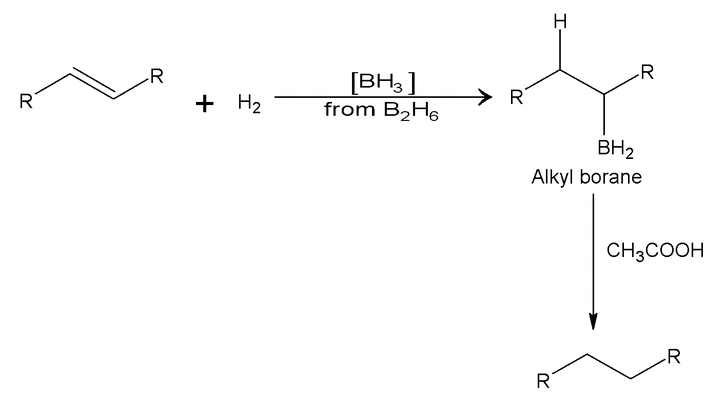
1. Conversion to alkanes (Heterogenous)
Relative rates of hydrogenation are as follows:
H2C = CH2 > RCH = CH2 > R2C = CH2, RCH = CHR > R2C > R2C = CR2
The rate decreases as steric hinderance increases.
2. Addition reactions
Addition of unsymmetrical reagents such as HX, HOX, H2O, H2SO4 etc to unsymmetrical alkenes such as propene occurs in accordance with Markonikov’s rule which states that the negative part of the addendum (adding regent) gets attached to that carbon atom of the double bond which has least number of hydrogen atoms
e.g.
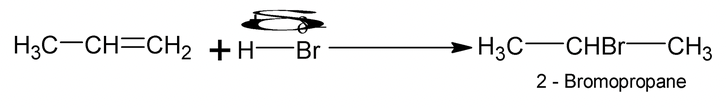
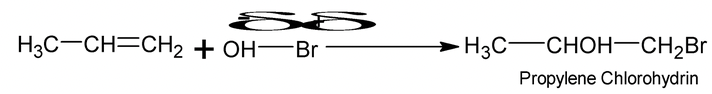
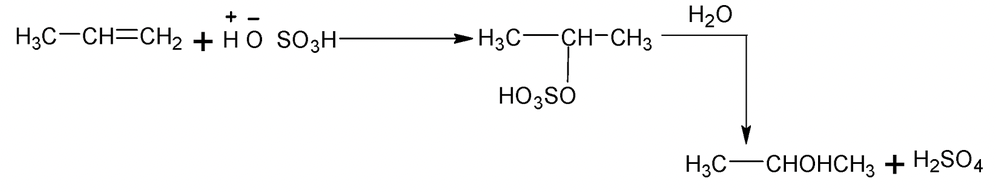
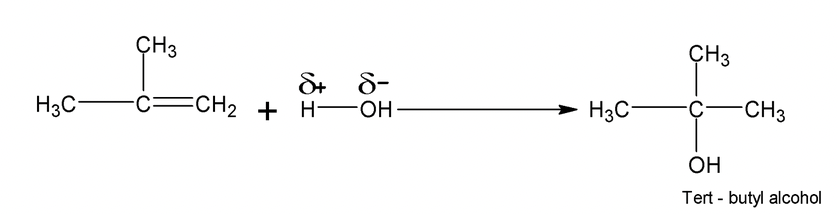
Carbocations are the intermediates and the major products always results from more stable carbocations. e.g.

In presence of peroxide, the addition of HBr to unsymmetrical alkenes occurs contrary to Markovnikov’s rule. e.g.
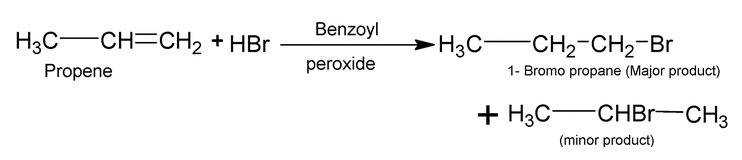
Anti markovnikov’s addition is generally called peroxide effect or kharasch effect. In presence of peroxides, the addition of HBr to unsymmetrical alkenes occurs by a free radical mechanism.
e.g.
Initiation: 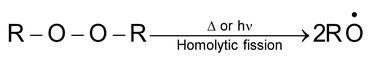
Peroxide
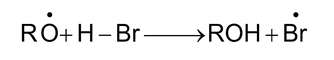
Propagation: (i) 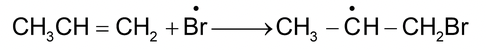
Free radical (more stable)
(ii) 
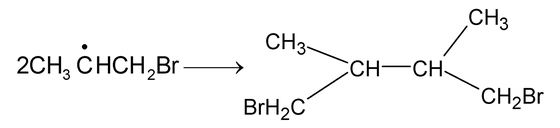
Termination: (i) 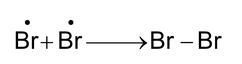
|
(ii) |
|
Note:
Peroxide effect is not observed with other halogen acids (HF, HCl or HI) since only HBr has both steps exothermic while with HCl second propagation step involving the reaction of carbon radical with HCl is endothermic and with HI, first propagation step involving the addition of iodine radical to alkene is endothermic.
Because of the presence of double bond alkene readily undergoes electrophilic addition reactions. Some important reactions of ethene are given below:
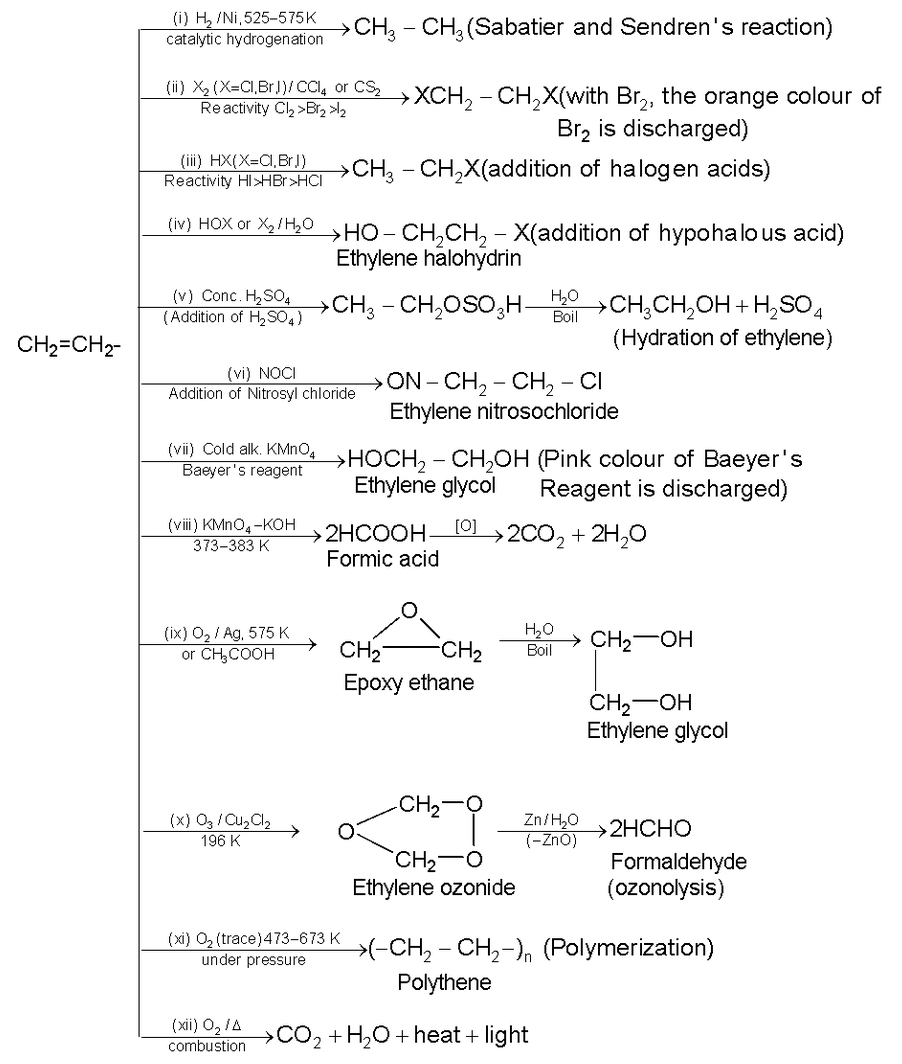
Other Resourceful Topics