
Mechanism Of Electrophilic Substitution Reactions
Hydrocarbon of Class 12
1.Nitration:
(i) Formation of electrophile 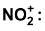
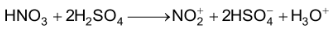
(base) (acid) (nitronium ion)
(ii) Electrophilic attack by 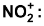
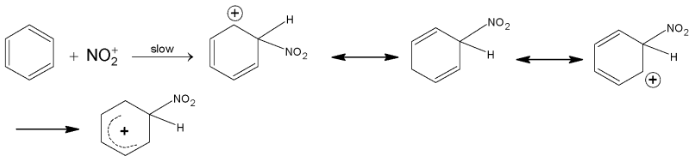
In the intermediate, attacked carbon atom changes its state of hybridization from trigonal to tetrahedral.

(iii) Loss of proton :
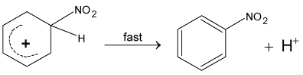
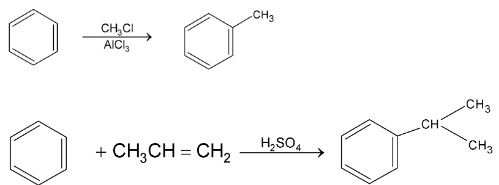
2. Friedel – Craft Alkylation:
This reaction involves the introduction of alkyl group into benzene ring in presence of catalyst. The alkylating agent may be R – X, ROH or alkene. The catalyst is a Lewis acid, 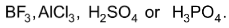
Mechanism:
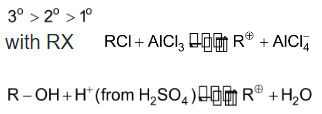
Step I: Formation of electrophile (carbonium ion) stability order of carbonium ion is
With ROH:
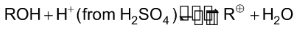
With alkene :

Step II :
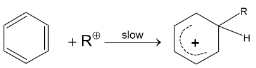
Step III :
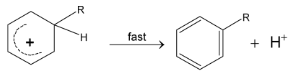
Examples:
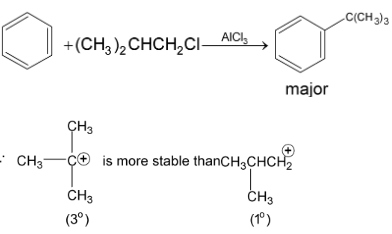
or 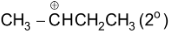 carbonium ion.
carbonium ion.
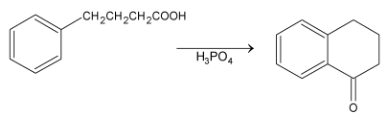
This is an example of intermolecular Friedel Craft alkylation. But if the side chain contains four or five carbons with halogen at the end then it may undergo intramolecular Friedel – Craft reaction.
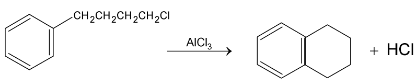
3.Friedel – Craft Acylation:
Replacement of 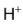 of benzene by
of benzene by 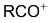 group using carboxylic acid, esters, acid chloride or acid anhydride as acylating agent in the presence of Lewis acid.
group using carboxylic acid, esters, acid chloride or acid anhydride as acylating agent in the presence of Lewis acid.
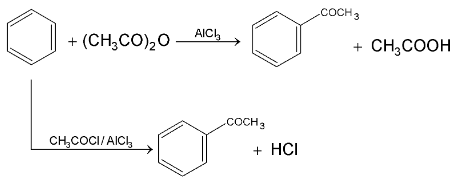
Mechanism:
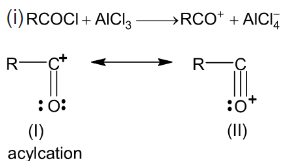
(II) is more stable than (I) because each atom has a complete octet.
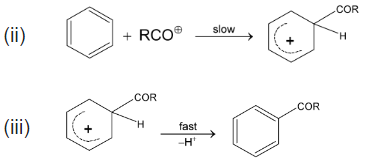
Examples:
Reactions of side chains
1.Halogenation:
|
|
With |
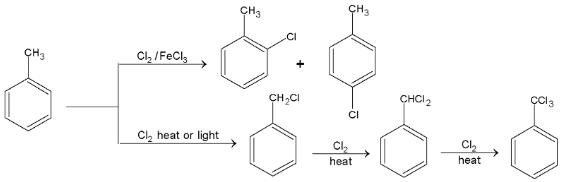

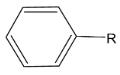
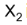 in presence of
in presence of 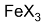 undergoes electrophilic substitution at o- and p- position and in the absence of
undergoes electrophilic substitution at o- and p- position and in the absence of 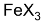 and in the presence of light or high temperature undergoes substitution in the side chain (free radical substitution).
and in the presence of light or high temperature undergoes substitution in the side chain (free radical substitution).