Alkynes
Hydrocarbon of Class 12
Nomenclature of alkynes:
In IUPAC system the compounds are named as alkynes in which the final – ane of the present alkane is replaced by the suffix – yne. The position of the triple bond is indicated by a number.

Propyne 3 – methyl – 1 – butyne
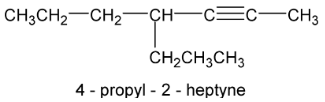
When both double and triple bonds are present, hydrocarbon is named as alkenyne.
eg : 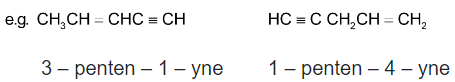
METHODS OF PREPARATIONS
1. Industrial source:

Calcium Carbide Ethyne
|
The double negative carbide ion |
|
|
which is strongly basic reacts with water to form acetylene
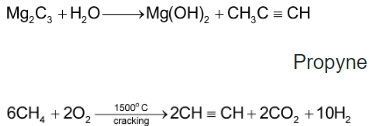
2.Kolbe’s method:
Electrolysis of concentrated solution of sodium or potassium salt of maleic or fumaric acid gives acetylene at anode
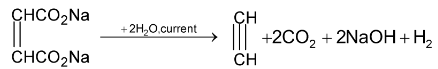
3.Dehydrohalogenation of 1, 2 – dihalides:
1, 2 – dihalides on treatment with alcoholic KOH gives alkynes by loss of two molecules of hydrogen halide, the intermediate being vinyl halide.
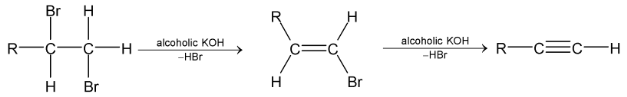
Sodamide  in liquid
in liquid  can be used instead of alcoholic KOH.
can be used instead of alcoholic KOH.

Mechanism:
Step I:
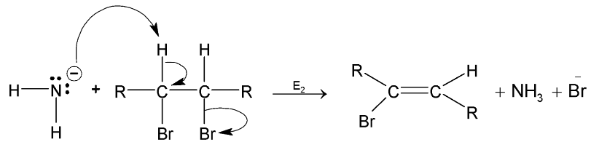
Step II:
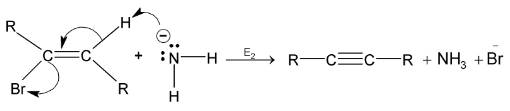
Elimination of the first molecule of HX results in the formation of vinyl halide – an alkene with halogen bonded to one of the carbons of the double bond. Strong base  is taken to make the step II easier for elimination.
is taken to make the step II easier for elimination.
4. Dehydrohalogenation of 1, 1 – dihalides:
1, 1 – dihalides (geminal) with alcoholic KOH or 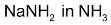 forms alkynes where intermediate is vinyl halide.
forms alkynes where intermediate is vinyl halide.
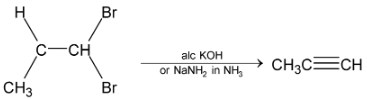
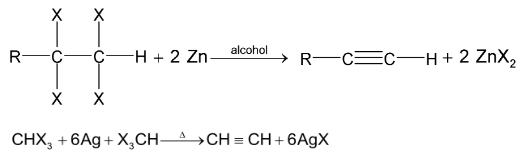
5. Dehalogenation of tetrahalides or trihalides:
6. Alkylation of acetylene and terminal alkynes:
i.e. an alkyne with 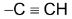 at the end of chain. This method is used to prepare higher alkynes.
at the end of chain. This method is used to prepare higher alkynes.
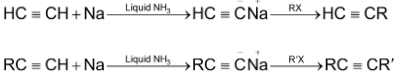
Terminal alkyne
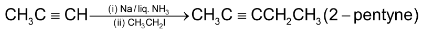
Note:
RX and  alkyl halides, since higher secondary and tertiary halides give mainly alkenes when they react with sodium salt of alkynes.
alkyl halides, since higher secondary and tertiary halides give mainly alkenes when they react with sodium salt of alkynes.
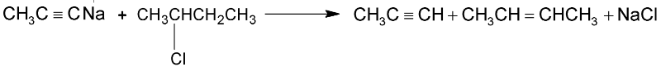
Alkylation can also be done by using Grignard’s reagent.
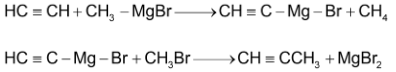
PHYSICAL PROPERTIES
(1.) Acetylene is a colourless gas with ethereal smell in pure state; b. pt.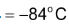
(2.) Liquified acetylene is explosive and burns with a luminous smoky flame due to high carbon content hence it is used for lighting purpose.
(3.) As the s – character of the orbitals involved in 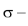 bonds increases from 25 percent
bonds increases from 25 percent 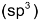 to 33.3 percent
to 33.3 percent 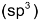 to 50 percent (sp), the electrons in those orbitals are on the average, close to carbon nucleus and this leads to a contraction in the internuclear distance.
to 50 percent (sp), the electrons in those orbitals are on the average, close to carbon nucleus and this leads to a contraction in the internuclear distance.
Bond lengths are in the order
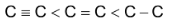
CHEMICAL PROPERTIES
1. Electrophilic addition reactions:
Alkynes undergo electrophilic addition reactions but less readily because of the following reasons:
(a) C – atoms of alkynes being sp – hybridized are more electronegative and hence hold the  more tightly.
more tightly.
(b)  of alkynes are more delocalized and hence are less easily available for reaction.
of alkynes are more delocalized and hence are less easily available for reaction.
(c) Vinyl carbocation formed after the addition of an electrophile to the alkyne is far less stable than the alkyl carbocations obtained by the addition of an electrophile to the alkene.
Some of the electrophilic addition reactions of alkynes are discussed below.
(i) Addition of halogens:
First trans – dihalides are formed which react further to form tetrahalides.
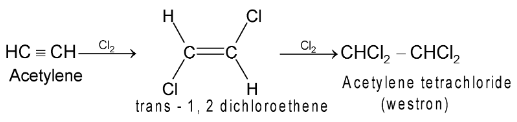
The order of reactivity with halogens is
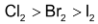
(ii) Addition of Hydrogen
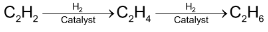
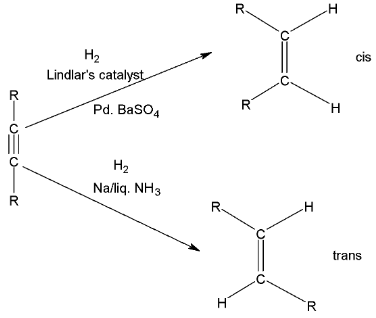
If the triple bond is not present at the end of chain of the molecule then the dialkyl acetylene may be catalytically reduced to cis and trans alkanes.
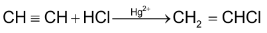
(iii) Addition of halogen acids
The addition is in accordance of markovnikov’s rule.

Because of ⎯ I effect of the bromine atom, the availability of the π - electrons in the first step is decreased and the addition is much slower as compared to ethylene. The reactivity is HI > HBr
> HCl. When passed through dilute HCl in the presence of Hg2+ as catalyst, acetylene forms vinyl chloride.
(iv) Addition of water
when passed into dilute H2SO4 at 600C in the presence of HgSO4 as catalyst acetylene adds on one molecule of water to form acetaldehyde.
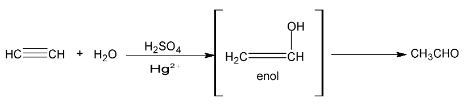
The homologous of acetylene forms ketone when hydrated.
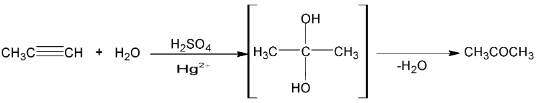
(v) Reaction with metals
A triply bonded carbon which is sp – hybridized has a high electronegativity and hydrogen atoms attached to it show appreciable acidity.
e.g. 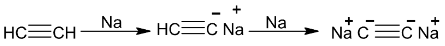
CH ≡ C−Na+ is also obtained by passing acetylene into a solution of sodium in liquid ammonia until the blue colour disappears.
Sodium acetylide can be used to prepare higher members of alkyne series.
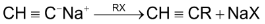
When acetylene is passed into an ammonical solution of Cu2Cl2 or AgNO3, cuprous acetylide Cu2C2 (red) or silver acetylide Ag2C2(white) is precipitated.
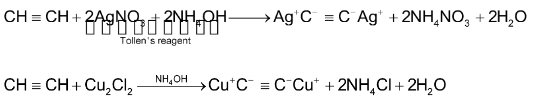
(vi) Ozonolysis
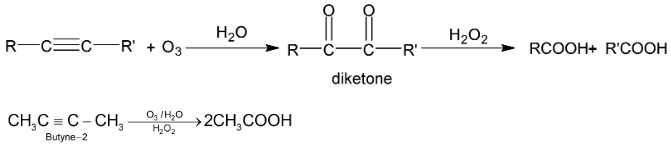
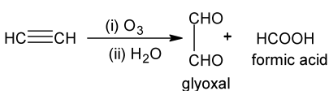
Acetylene is exceptional that it gives glyoxal as well as formic acid.
(vii) Polymerisation
When passed through a heated tube (Fe, Cu or Ni), acetylene changes to small extent of benzene.
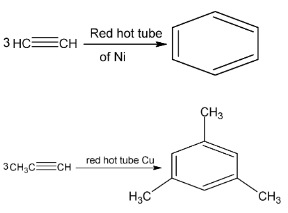
1, 3 , 5 – trimethyl benzene (mesitylene)
When passed into a solution of Cu2Cl2 in NH4Cl, acetylene gives vinyl acetylene.

But – 3 – en – 1 – yne
Compounds containing both double and triple bonds are named as alkenynes.
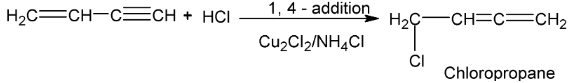
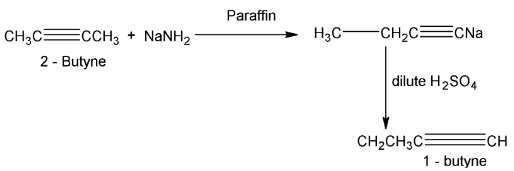
(viii) Isomerization
When alkynes are heated with NaNH2 in an inert solvent triple bond moves towards the end of chain.
Acidity of Alkynes
⎯C ≡ CH bond has considerable ionic character due to resonance.
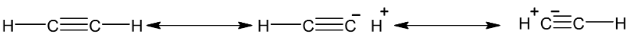
The electronegativity of a carbon atom depends on the number of bonds by which it is attached to its neighbouring carbon. The carbon with electronegativity of an sp2 hybridised carobn atoms is greater than that of sp3 hybridised carbon atom and the electronegativity of an sp hybridized carbon atom is further greater than that of sp2 carbon atom. More the s – character a bond has more electronegative is the carbon atom. Thus the attraction for electrons by hybridized carbon will be
sp > sp2 > sp3
Hence greater the electronegativity of an atom, more readily it accommodates a negative charge.
∴ Stabilities of the following carbanions follows the order.
CH ≡ C− > CH2 = CH− 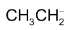
Aromatic Hydrocarbons
The term aromatic (Greek; aroma means fragrance) was first used for compounds having pleasant odour although the structure was not known. Now the term aromatic is used for a class of compounds having a characteristic stability despite having unsaturation. These may have one or more benzene rings (benzenoid) or may not have benzene ring (non-benzenoid). Benzenoid compounds include benzene and its derivatives having aliphatic side chains (arenes) or polynuclear hydrocarbons, eg. naphthalene, anthracene, biphenyl etc.
Structure of Benzene
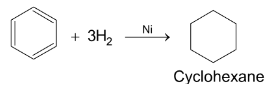
Benzene has been known since 1825 when it was first isolated by Michel Faraday. Form elemental analysis and molecular mass determination, it was found that the molecular formula of benzene is C6H6 indicating high unsaturation. However, benzene does undergo addition reactions in contrast to unsaturated hydrocarbons, although it mainly undergoes substitution reactions.
In 1865 Friedrich August Kekule proposed a ring structure for benzene (I). However, many alternative structures have been proposed from time to time by different workers. (II-IV).
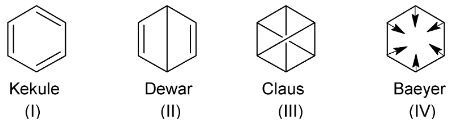
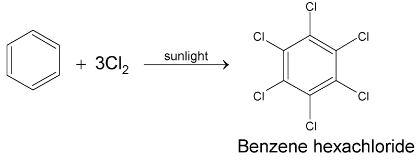
Then main objection against Kekule structure was that it should yield two ortho disubstituted products when it reacts with bromine. However, experimentally benzene was found to yield only one product.
Kekule removed this objection by proposing that the double bonds in benzene are continuously oscillating back and forth between two adjacent positions. Since positions of double bonds are not fixed, only one product is formed. This structure came to be known as Kekule’s dynamic formula, which formed the basis for the present electronic structure of benzene.
Modern Concept
Stability of Benzene (Resonance)
Benzene resists addition whereas it readily undergoes substitution reactions, like nitration, halogenation etc. This indicates that benzene is more stable than the hypothetical cyclohexatriene molecule. This has been proved by the fact that the enthalpies of hydrogenation and combustion of benzene are lower than expected. Enthalpy of hydrogenation is the change in enthalpy when one mole of an unsaturated compound is hydrogenated. It has been found experimentally that enthalpy of hydrogenation for disubstituted alkenes, R-CH=CH-R varies between 117-125 kJ mol−1. Accordingly, the values for cyclohexene and cyclohexa-1, 3-diene and hypothetical cyclohexa-1, 3, 5-triene were calculated compared with their experimental values.
While the experimental values of enthalpy of hydrogenation for cyclohexene is similar to the value expected, the variation in the case of cyclohexa-1, 3-diene is small and is due to delocalization. The expected value of enthalpy of hydrogenation of benzene is much higher than the corresponding calculated value for hypothetical cyclohexa-1, 3, 5-triene indicating that benzene does not have this type of structure.
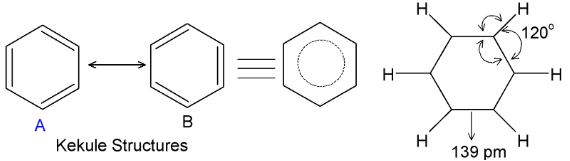
X-ray studies show that it is planar molecule and that all six C−C bonds in benzene are of equal length (139 pm), intermediate between C−C single bond (154 pm) and C=C (134 pm). All six carbons are sp2 hybridized and all bond angles are 120o. Benzene is a hybrid of various resonating structures, the two Kekule structures A and B, being the main contributing forms.
Aromaticity in Benzene and Related Systems
After the structure of benzene was established, the term aromatic was adapted for such compounds which despite having π bonds (unsaturation) resist addition and instead undergo substitution. The aromaticity in benzene is attributed to the six delocalized pi electrons in the coplanar carbon hexagon. When a bonding orbital is not restricted to two atoms but is spread over more than two atoms, e.g. six in benzene, such bonding orbitals are said to be delocalized. Delocalisation results in greater stability.
The modern theory of aromaticity was advanced by Eric Huckel 1931. Aromaticity is a function of electronic structure. Any polynuclear compound, heterocyclic rings or cyclic ions may be aromatic if these have a specific electronic structure. The important features of the theory are
1. Delocalization: Complete delocalization of π electron cloud of the ring system is a necessary requirement for aromatic character.
2. Planarity: Complete delocalization of π-electron cloud is possible only if the ring is planar. This is the reason that benzene is aromatic but cyclooctatetraene is not, since the latter is not a planar molecule.
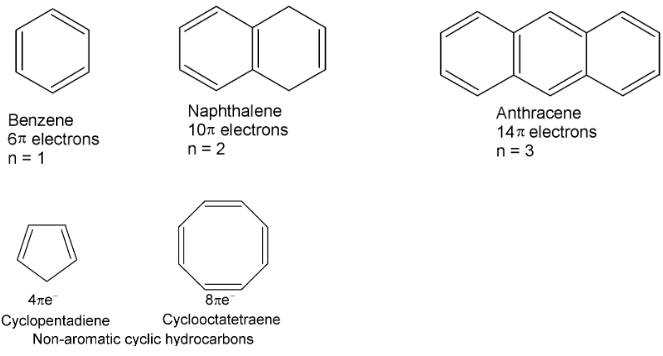
Huckel’s rule or (4n + 2)π electron rule: The rule states that in a conjugated, planar, cyclic system if the number of delocalized π-electrons is (4n + 2) where n is an integer, i.e., 1, 2, 3 etc. Benzene, naphthalene, anthracene and phenanthrene are aromatic as they contain (4n + 2)π electrons i.e. 6, 10, 14, π electrons in a conjugated cyclic system. The cyclopentadiene and cyclooctatetraene are non-aromatic as instead of (4n + 2)π e− these have 4n π e−. Moreover, they are non-planar.
Benzene was first isolated by Faraday from cylinders of compressed illuminating gas obtained from the pyrolysis of whale oil.
Benzene was first synthesised by Berthelot by passing acetylene through a red hot tube.

In the laboratory it was first prepared by heating benzoic acid or phthalic acid with calcium oxide.
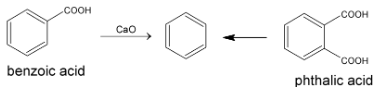
PROPERTIES
(i) It is a colourless liquid with boiling point of 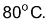
(ii) It is inflammable, burning with a smoky flame due to high carbon content.
(iii) It is slowly attacked by acid permanganate to form 

|
(iv) |
|
|
(v) |
|
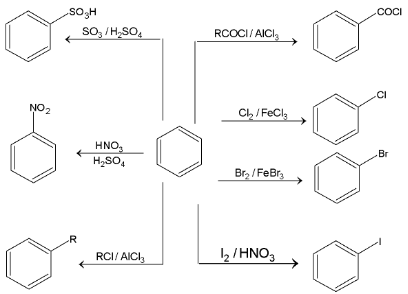
Reaction of benzene:
Major reactions of benzene ring are electrophilic substitution reactions.