Mechanism Of Some Important Reactions Of Alkenes
Hydrocarbon of Class 12
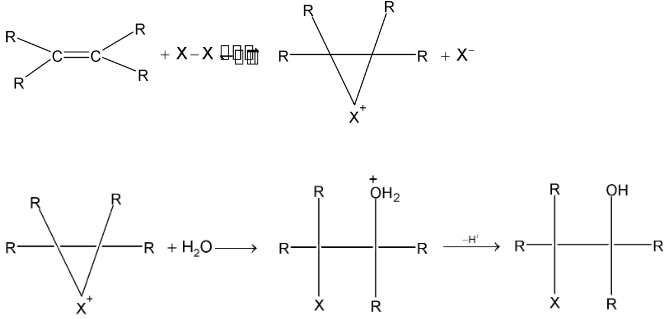
1. Mechanism of halogen addition:
The mechanism proposed is an ionic mechanism.
In the first step the exposed electrons of the 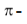 bond of the alkene attacks the halogen in the following way:
bond of the alkene attacks the halogen in the following way:
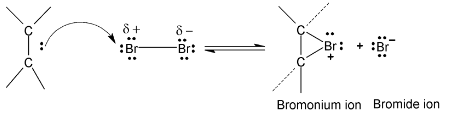
As 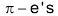 of the alkene approach the bromine molecules, the electrons of bromine – bromine bond drift to make bromine molecule polarised. The more distant bromine develops a partial negative charge and nearer bromine becomes partially positive. Polarization weakens the bond and cleaves it heterolytically.
of the alkene approach the bromine molecules, the electrons of bromine – bromine bond drift to make bromine molecule polarised. The more distant bromine develops a partial negative charge and nearer bromine becomes partially positive. Polarization weakens the bond and cleaves it heterolytically.
In second step, one of the bromide ions predicted in step I attacks one of the carbon atoms of the bromonium ion. The nucleophilic attack results in the formation of a vicinal dibromide by opening the three – membered ring.
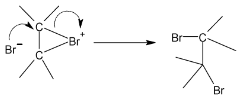
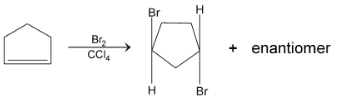
On reaction of cyclopentane with bromine in 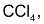 anti – addition occurs and the products of the reaction are trans – 1, 2 dibromocyclopentane enantiomers (as a race`mate)
anti – addition occurs and the products of the reaction are trans – 1, 2 dibromocyclopentane enantiomers (as a race`mate)
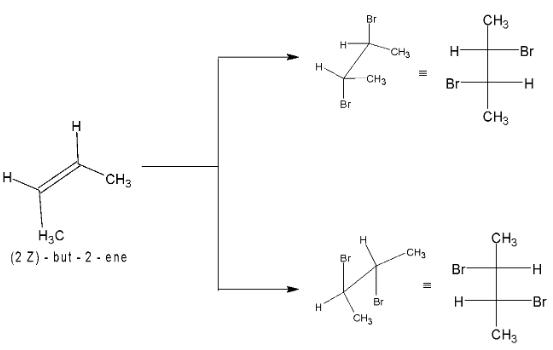
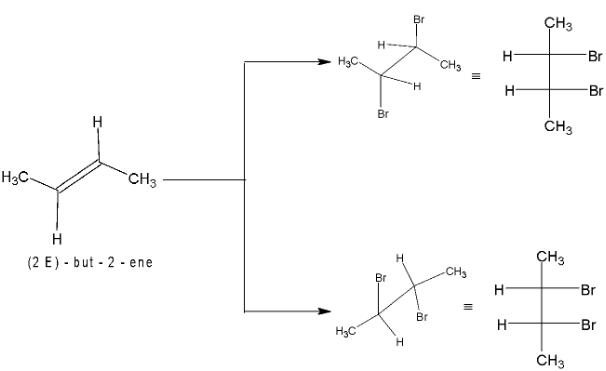
Addition of bromine to cis – 2 – butene gives racemic form of 2, 3 – dibromobutane.
Bromine adds to trans – 2 – butene to form meso compound, thus the reaction is stereospecific in nature.
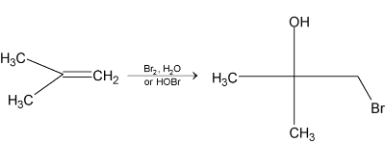
2. Mechanism of halohydrin formation
It can be explained by the following mechanism:
If the alkene is unsymmetrical, the halogen ends up on the carbon atom with greater number of hydrogen atoms.
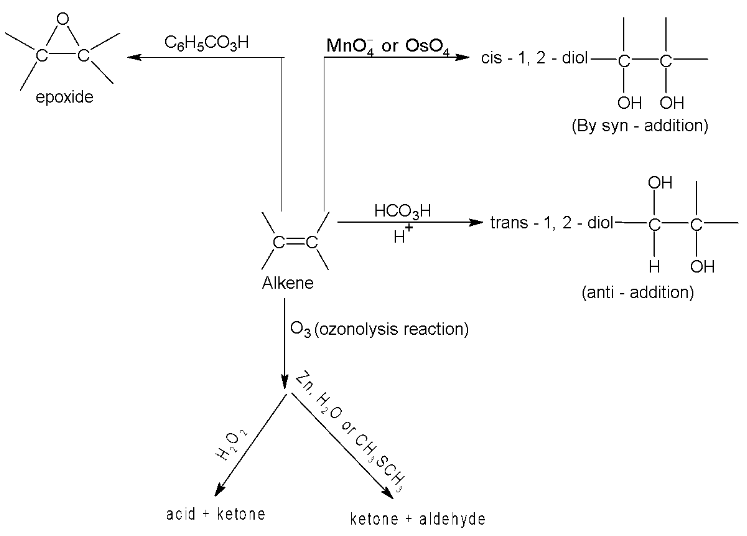
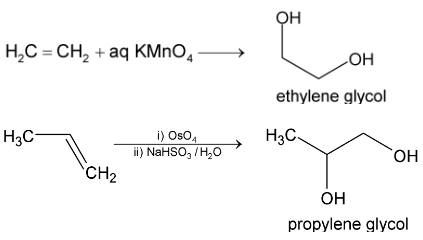
3. Syn - hydroxylation
Hydroxylation with permanganate is carried out by reaction at room temperature. It’s a good method for the synthesis of 1, 2 – diols.
Mechanism in both cases involves formation of cyclic intermediates, then in several steps, the cleavage at oxygen – metal bond takes place producing glycol and 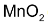 or Os metal.
or Os metal.
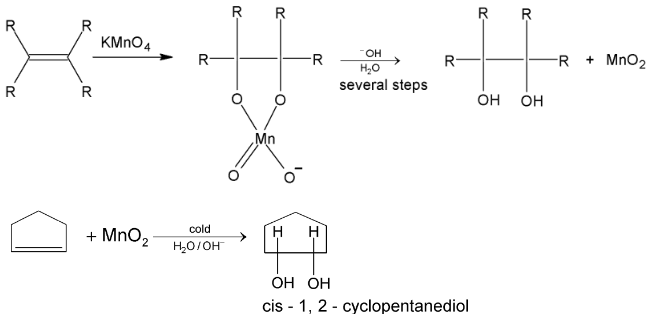
Cis – 2 – butene when treated with cold alkaline 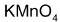 gives meso glycol and trans – 2 – butene gives racemate.
gives meso glycol and trans – 2 – butene gives racemate.
4. Oxidation reactions of alkenes
(i) With cold dilute 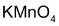 (Baeyer’s reagent) alkenes give 1, 2 – glycols.
(Baeyer’s reagent) alkenes give 1, 2 – glycols.
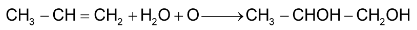
Propene From  Propylene glycol
Propylene glycol
(ii) With hot alkaline 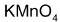
Cleavage of C = C bond takes place leading to formation of carboxylic acids, ketones and 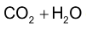 depending upon structure of alkene.
depending upon structure of alkene.
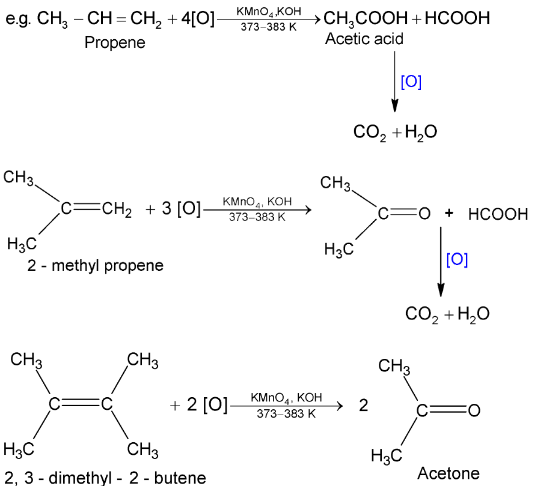
Hence by identifying the products formed during alkaline  oxidation, it is possible to determine the position of the double bond in an alkene molecule.
oxidation, it is possible to determine the position of the double bond in an alkene molecule.
(iii) With ozone alkenes first give ozonides which upon reductive cleavage with Zn dust and  gives aldehydes / ketones or a mixture of these depending upon the structure of alkene.
gives aldehydes / ketones or a mixture of these depending upon the structure of alkene.
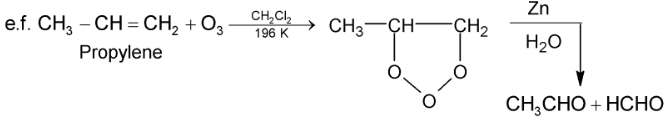
This two step – conversion of alkene into ozonide followed by decomposition with  to give aldehydes / ketones or a mixture of these is called reductive ozonolysis.
to give aldehydes / ketones or a mixture of these is called reductive ozonolysis.
If however ozonide is decomposed with 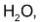 the initially formed aldehydes are further oxidized to the corresponding acids by
the initially formed aldehydes are further oxidized to the corresponding acids by 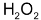 produced in the reaction. This is called oxidation ozonolysis.
produced in the reaction. This is called oxidation ozonolysis.
The oxidation reactions of alkenes are summed up as follows: