Toluene
Hydrocarbon of Class 12
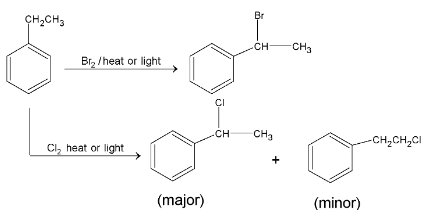
For side chain halogenation, case of abstraction of hydrogen atom is as follows:
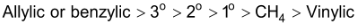
the stability order of free radicals is
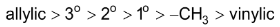
Bromine is more selective than chlorine.
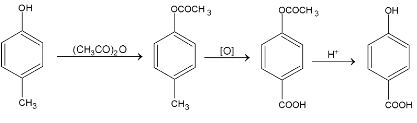
2. Oxidation:
Benzene ring is usually very resistant to oxidation, hence the side chain is always attacked. Whatever the length of side chain the ultimate oxidation product is benzoic acid.
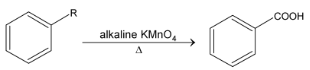
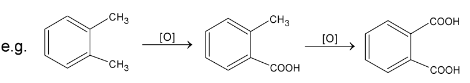
When two side chains are present, it is possible to oxidize them at same time.
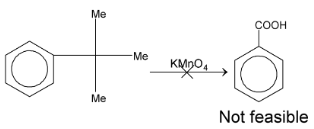
But, if the C attached to benzene ring does not have any hydrogen then it will not give benzoic acid.
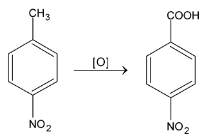
When an electron withdrawing group (-I and / or –R) is present, the ring is stable and the result of oxidation is a substituted benzoic acid.
If –OH or 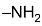 is present, the ring is very sensitive to oxidation and is largely broken down, whatever be the nature of oxidizing agent. Ring rupture can be prevented by protection of the group.
is present, the ring is very sensitive to oxidation and is largely broken down, whatever be the nature of oxidizing agent. Ring rupture can be prevented by protection of the group.