
Carbocations (Earlier Called As Carbonium Ions)
IUPAC & GOC of Class 12
Carbocations
Carbocations are the key intermediates in several reactions and particularly in nucleophilic substitution reactions and electrophilic addition reaction.
(a) Structure:
Generally in the carbocations the positively charged carbon atom is bonded to three others atoms and has no nonbonding electrons. It is sp2 hybridized with a planer structure and bond angles are of about 1200. There is a vacant unhybridised p orbital which (e.g in the case of 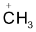 lies perpendicular to the plane of C ⎯ H bonds.
lies perpendicular to the plane of C ⎯ H bonds.
![]()
(b) Stability:
There is an increase in carbocation stability with additional alkyl substitution. Thus one finds that addition of HX to three typical olefins decreases in the order
(CH3)2C = CH2 > CH3 ⎯ CH = CH2 > CH2 = CH2
This is due to the relative stabilities of the carbocations formed in the rate determining step which in turn follows from the fact that the stability is increased by the electron releasing methyl group (+I), three such groups being more effective than two, and two more effective than one.
![]()
Stability of carbocations 30 > 20 > 10 > ![]()
Electron release: Disperses charge, stabilizasion.
Further, any structural feature which tends to reduce the electron deficiency at the tricoordinate carbon stabilizes the carbocation. Thus when the positive carbon is in conjugation with a double bond. The stability is more. This is so, due to resonance the positive charge is spread over two atoms instead of being concentrated only on one. This explains the stability associated with the allylic cations. The benzylic cations are stable, since one can draw canonical forms as for allylic.
![]()
The benzyl cation stability is affected by the presence of substituents on the ring. Electron donating p – methoxy and p – amino group stabilize. The carbocation by 14 and 26 kcal/mole, respectively. The electron withdrawing groups like e.g, p – nitro destabilize by 20 kcal/mol.
A heteroatom with an unshared pair of electrons when present adjacent to the cationic centre strongly stabilizes the carbocation. The methoxy methyl cation has been obtained as a stable solid ![]() Fcylopropylmethyl cations are even more stable than the benzyl cations. This special stability is a result of conjugation between the bent orbitals of the cyclopropyl ring and the vacant p orbital of the cationic carbon. The carbocations are planar is shown by the fact these are difficult or impossible to form at bridgeheads, where they can not be planar.
Fcylopropylmethyl cations are even more stable than the benzyl cations. This special stability is a result of conjugation between the bent orbitals of the cyclopropyl ring and the vacant p orbital of the cationic carbon. The carbocations are planar is shown by the fact these are difficult or impossible to form at bridgeheads, where they can not be planar.
The stability order of carbocation is explained by hyperconjugation. In vinyl cations (CH2 = ![]() ) resonance stability lacks completely and therefore are very much less stable.
) resonance stability lacks completely and therefore are very much less stable.
Stability ∝ hyperconjugated structures ∝ number of α hydrogen.
