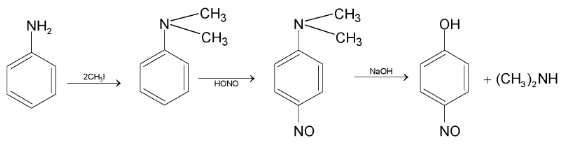
Distinguish Tests Between Nitroalkanes And Alkyl Nitrities
Nitrogen and Other P block Elements of Class 12
Distinguish Tests Between Nitroalkanes And Alkyl Nitrities
1. Nitroalkane on reduction with 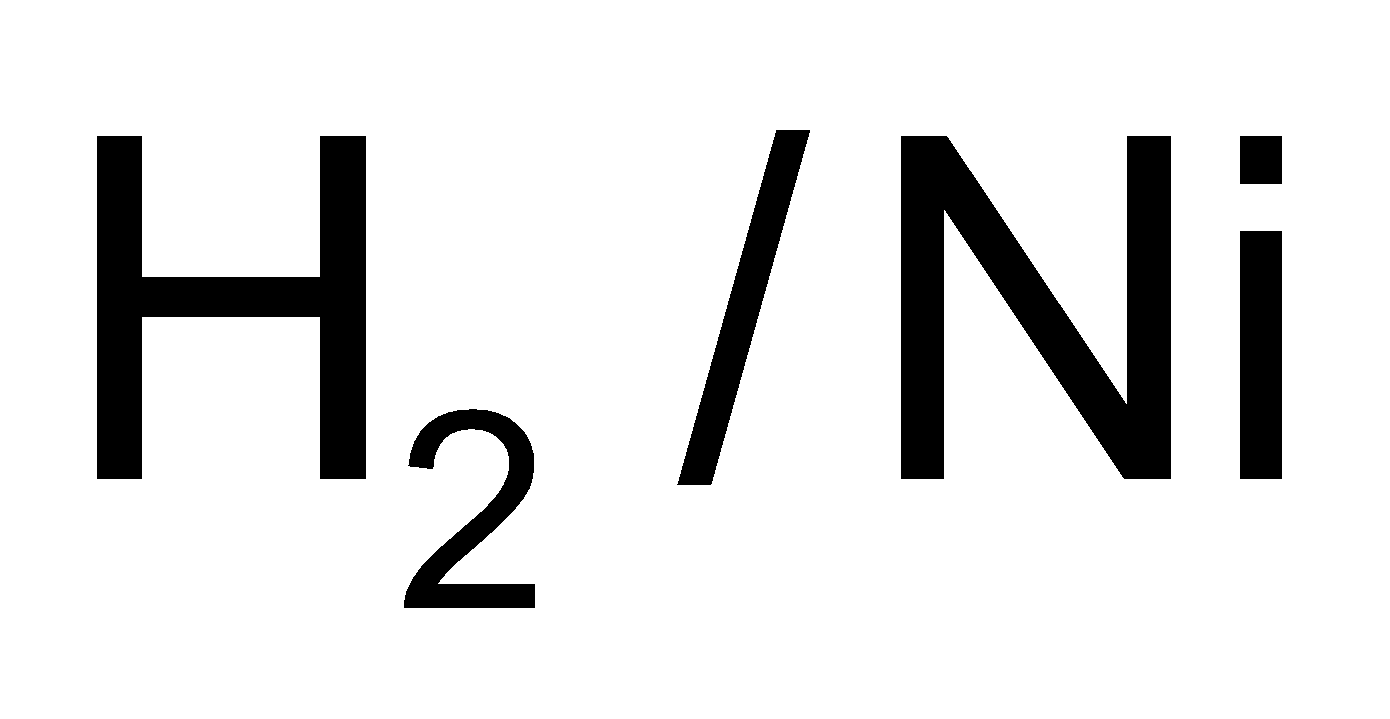 produce
produce  amines while alkyl nitrites produce alcohols and
amines while alkyl nitrites produce alcohols and 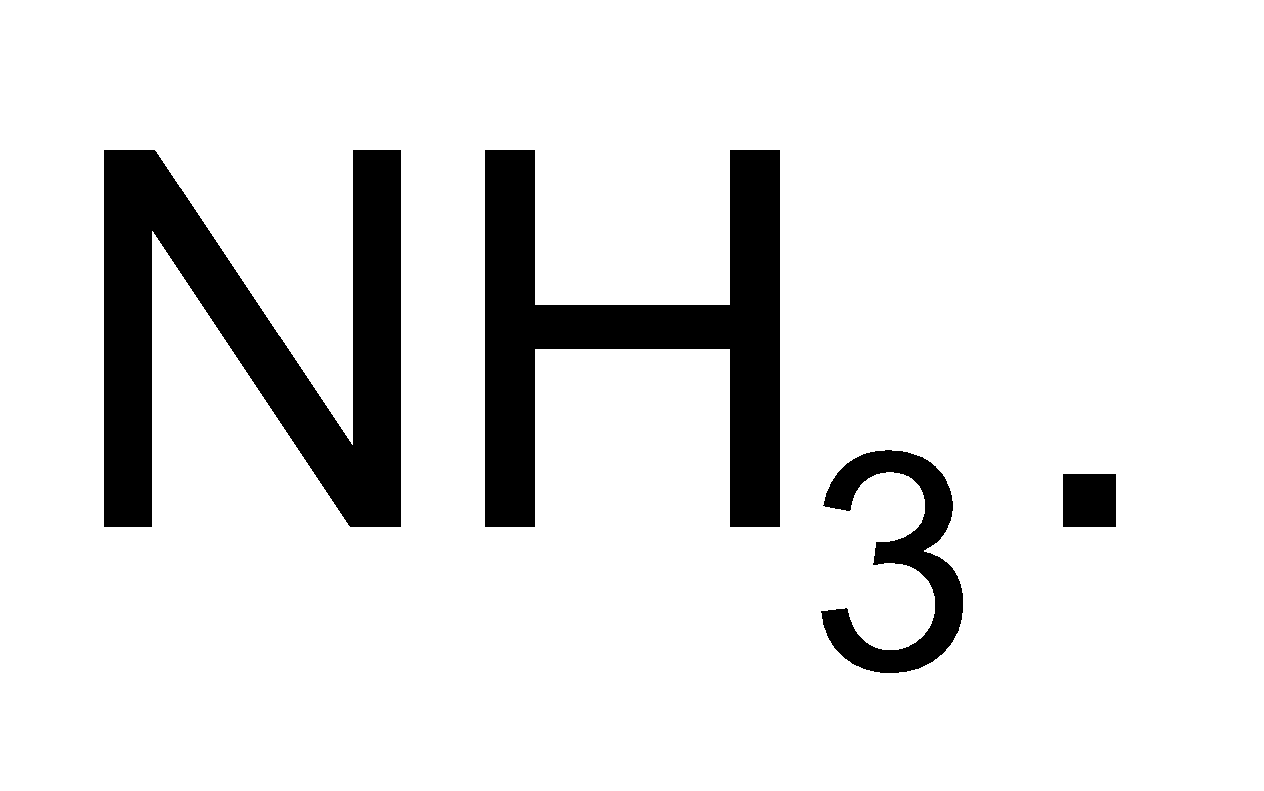
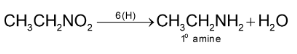
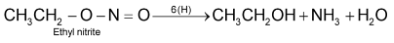
2. Nitroalkanes do not get hydrolysed in basic conditions while nitrites produce alcohols
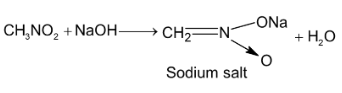
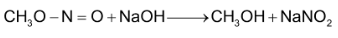
Illustration 3. Nitrobenzene, but not benzene, is used as a solvent for Friedel – Craft alkylation of bromobenzene. Why?
Solution: If during alkylation of bromobenzene benzene is used as a solvent, alkylation of benzene will take place because benzene is more reactive in electrophilic substitution reactions than bromobenzene. On the other hand, nitrobenzene does not under goes Friedel – Craft reaction and can be used as solvent for alkylation of bromobenzene.
Illustration 4. An organic compound (A) C2H5NO2 gave on hydrolysis an alcohol (B) of molecular mass 46 and an acid. On reduction it gave the same alcohol (B) and NH3 gas. What is (A)?
Solution: (i) Molecular formula of A suggests that it is either.
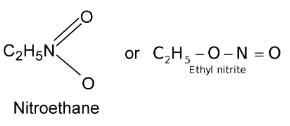
(ii) Since (A) undergoes hydrolysis to give (B) and an acid, it is ethyl nitrite C2H5O⎯N =O.
Reaction:
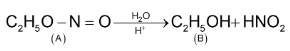
Molecular mass of (B) is 46

Illustration 5. Convert benzene to m-nitro aniline.
| Solution: |
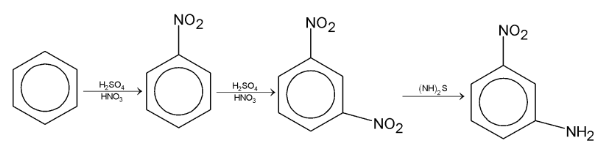 |
AMINES
Amines are derivatives of  They are represented by general formula
They are represented by general formula 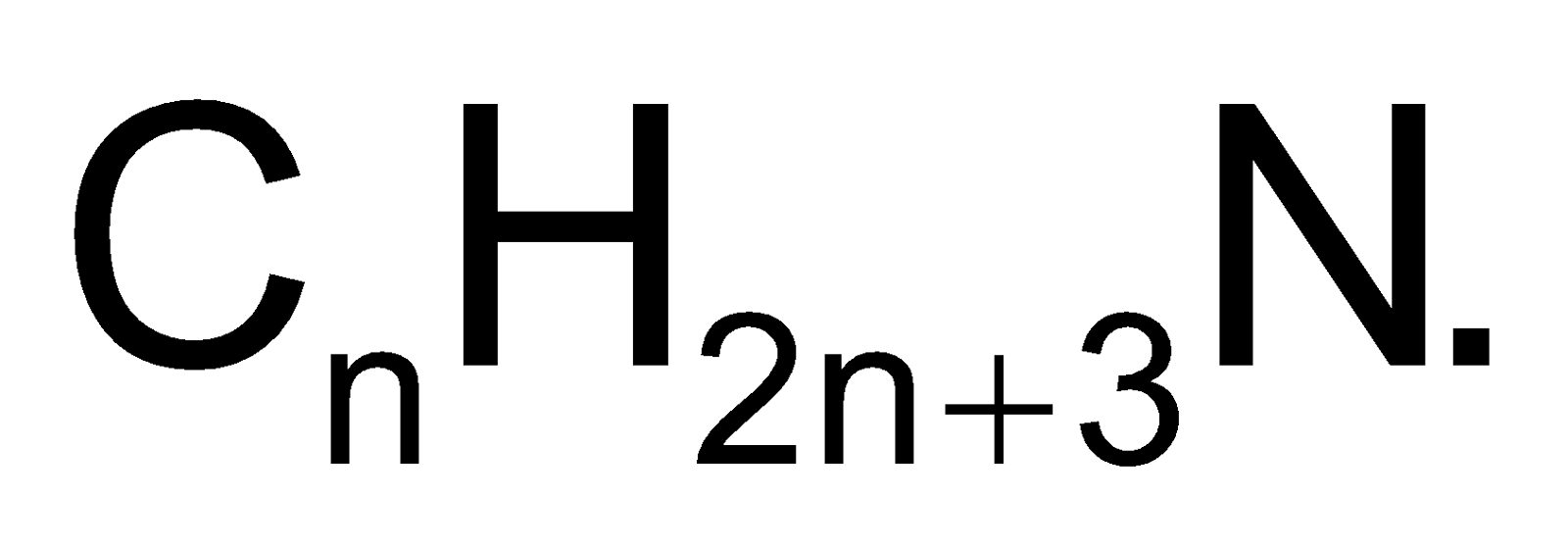
General methods of preparation:
1. Alkylation of  with alkyl halides or alcohols:
with alkyl halides or alcohols:
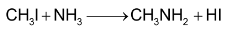
This reaction is called Hofmann’s Method. Exhaustive methylation is as follows:
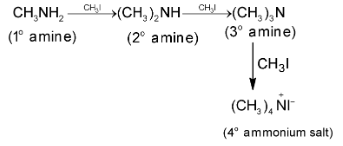
Aryl halides show low reactivity for this reaction.
2. By the action of ammonia on alcohol:
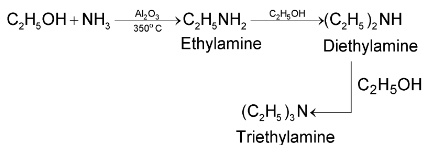
This method yields a mixture of  amines and
amines and  salts which are separated from each other by means of Hinsberg method, Hofmann method and fractional of distillation. However,
salts which are separated from each other by means of Hinsberg method, Hofmann method and fractional of distillation. However,  amines can be prepared in good yield by using excess of ammonia.
amines can be prepared in good yield by using excess of ammonia.
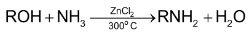
Illustration 6. Which of the following compounds exists as non resolvable racemic mixture?
| (A) |
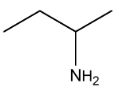 |
(B) |
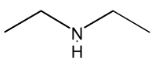 |
| (C) |
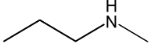 |
(D) |
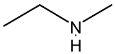 |
Solution: 3° amine contain all three alkyl groups different give non resolvable mixture.
Hence (D) is correct
Illustration 7. The statement not correct for piperidine is
(A) it is heterocyclic compound (B) it is homocyclic compound
(C) it is more basic than pyridine (D) it is more basic than aniline
Solution: Piperidine is heterocyclic compound.
Hence (A), (C) and (D) are correct and (B) is wrong.
Illustration 8. N-Ethyl-N-methyl propanamine does not show optical activity why?
| Solution: |
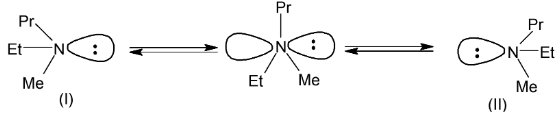 |
The energy difference between I and II is very small, these are rapidly interverted into each other hence are non resolvable. This is called nitrogen inversion or umbrella effect.
Methods yielding only  AMINES:
AMINES:
1. By reduction of nitroalkanes:
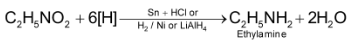
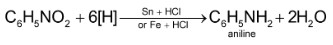
2. Mendius reduction of alkyl cyanides:
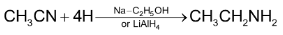
Ethylamine
Reduction of alkyl isocyanides with 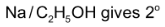 amines eg.
amines eg.
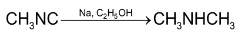
3. By reduction of amides and oximes:
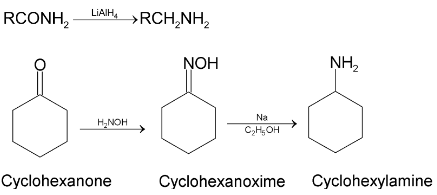
4. By Hofmann bromamide reaction:
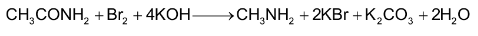
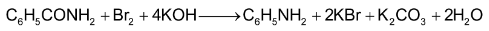
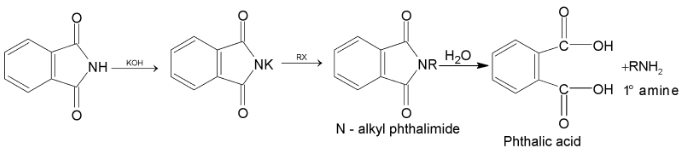
5. By Gabriel phthalimide reaction:
Potassium phthalimide formed after reaction of phthalimide with KOH, on heating with alkyl halide gives N – alkyl phthalimide. This on hydrolysis with 20% hydrochloric acid under pressure gives 1° amine.
| Illustration 9. |
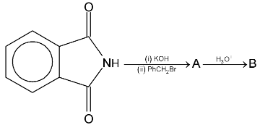 |
The product B is
| (A) |
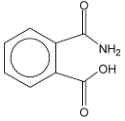 |
(B) | PhCH2NH2 |
| (C) |
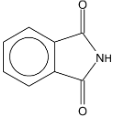 |
(D) |
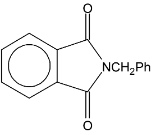 |
Solution: Gabrial pthalamide reaction.
Hence (B) is correct.
Illustration 10. Which of the following amines can not be prepared by Gabriel Phthalimide. synthesis?
- CH3CH2NH2
- (C2H5)2NH
- CH3CH2CH2NH2
Solution: (C2H5)2NH is a secondary amine and hence can not be prepared by this method since this method can be used to get only primary amines.
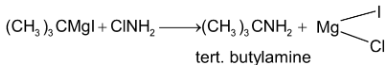
6. By action of chloramine on Grignard’s reagent:
7. By decarboxylation of amino acids:
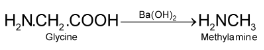
8. By Wurtz method:
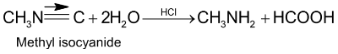
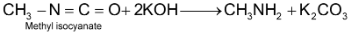
9. Schmidt reaction:
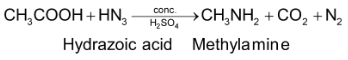
Methods giving 2° amines only:
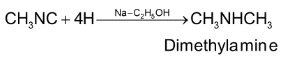
1. By reduction of alkyl isocyanide with sodium and ethanol
2. By heating an alcoholic solution of 1° amine with alkyl halide
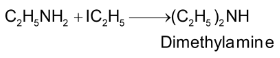
3. By hydrolysis of p – nitroso dialkyl aniline with boiling alkali