Structures
Nitrogen and Other P block Elements of Class 12
Evidences show that nitrogen is attached to one of the oxygen atoms by a double bond and to the other by a dative bond. The resonance hybrid is shown as under which confirms the spectroscopic evidence that both nitrogen – oxygen bonds have same bond length.
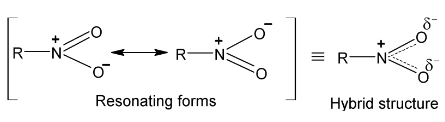
Out of three sp² hybrid orbitals of nitrogen one overlaps with alkyl group and two with oxygens while the unhybridized p orbital of N – atom containing a pair of electrons and lying perpendicular to the plane of hybrid orbitals overlaps sideway with half filled 2 p – orbitals of two oxygen atoms. This forms π-bond above and below the plane of molecule.
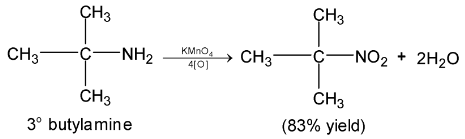
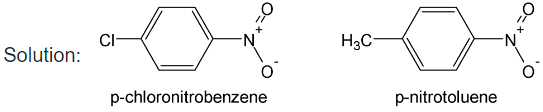
Illustration 39. p-chloronitrobenzene has less dipole moment (2.4D) than p-nitrotoluene. Explain.
|
Solution: |
|
In p – chloronitrobenzene, the two vector dipole moment act opposite to each other which partially cancel each other. On the other hand, in p – nitrotoluene, the two vectors acts in the same direction to give additive dipole resultant.
METHODS OF PREPARATION:
(i) From alkyl halides:
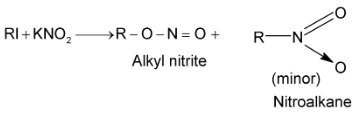
Alkyl halides react with silver nitrite in ethanolic solution to give nitro compounds. Alkyl nitrite is formed in minor quantity. This reaction is used to prepare 1° nitro compounds primarily while 2° and 3° halides give major proportion of alkenes due to β – elimination. Contrary to this alkali nitrites give alkyl nitrites as major product. This is due to ionic nature of alkali nitrite.
But if the reaction is carried out in solvents like DMF or DMSO, then even 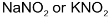 give good yield (about 60%) of nitro compound.
give good yield (about 60%) of nitro compound.
Reactions:
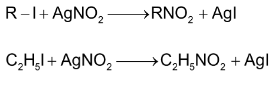
Nitroethane
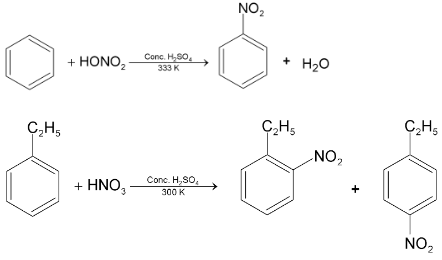
(ii) Nitration:
Nitro derivatives of aromatic compounds like nitrobenzene are produced when benzene is allowed to react with nitrating mixture. .
.
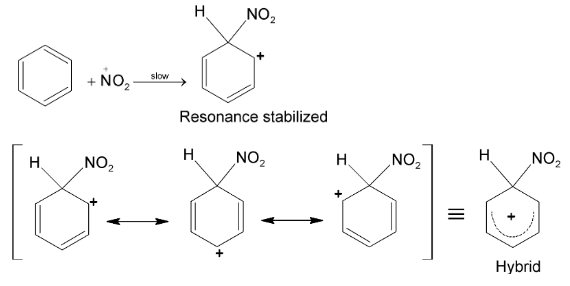
Mechanism:
Generation of nitronium ion
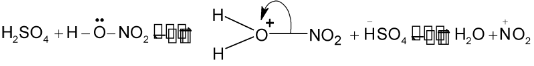
Attack of 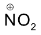 on benzene molecule
on benzene molecule
Loss of proton:
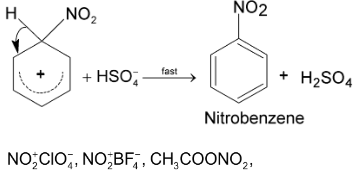
etc are other nitrating agents used.
Direct nitration of alkane involves vapour phase nitration at high temperature.

low yield
Problem faced in the method is that at such high temperature, a mixture of nitro alkenes is formed due to C – C cleavage.
e.g. 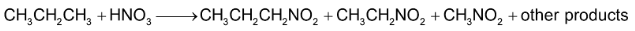
(iii) From amines:
3° nitroalkanes can be produced as follows –