
Properties Of Nitro Compounds
Nitrogen and Other P block Elements of Class 12
Properties Of Nitro Compounds
Nitroalkanes are colourless liquids with pleasant smell while aromatic nitro compounds have characteristic odour. Nitro alkanes are sparingly soluble in water, are highly polar with strong dipole – dipole interactions due to which they have high boiling points. Most of nitroalkanes are quite stable and can be distilled without decomposition but alkyl nitrites are unstable and explode on heating. Nitro compounds like nitrobenzene, o – nitrophenol are steam volatile and can be purified by steam distillation.
Reactions of nitro alkanes are given below:
Reduction:
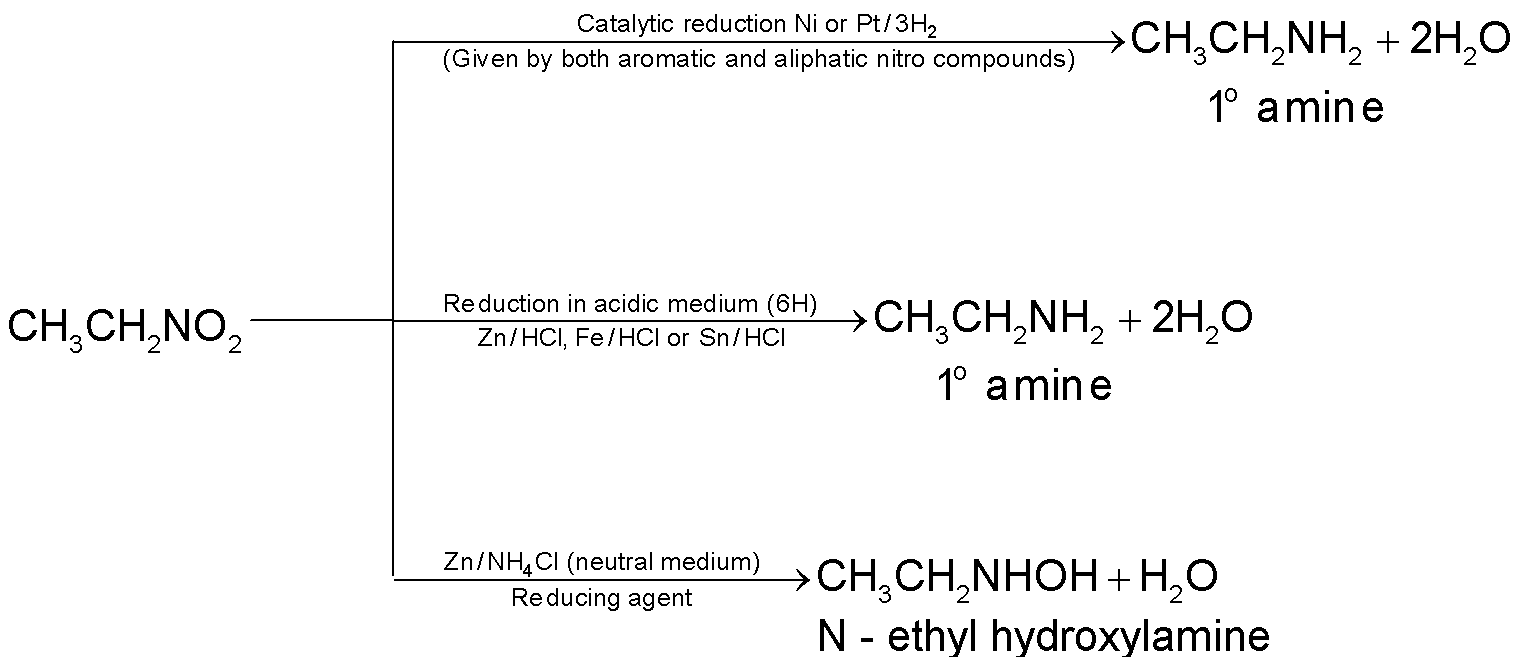
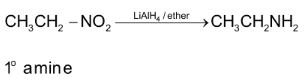
Note:
Hydroxylamine on warming with ammonical silver nitrate solution (Tollen’s reagent) gets oxidized to nitroso compound and reduces Tollen’s reagent to metallic silver. This reaction is a test for nitro compounds and is known as Baker – Milliken’s test.
Aromatic nitrocompounds give azo compounds on above reduction
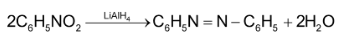
Aromatic nitro compounds undergo bimolecular reduction in alkaline medium.
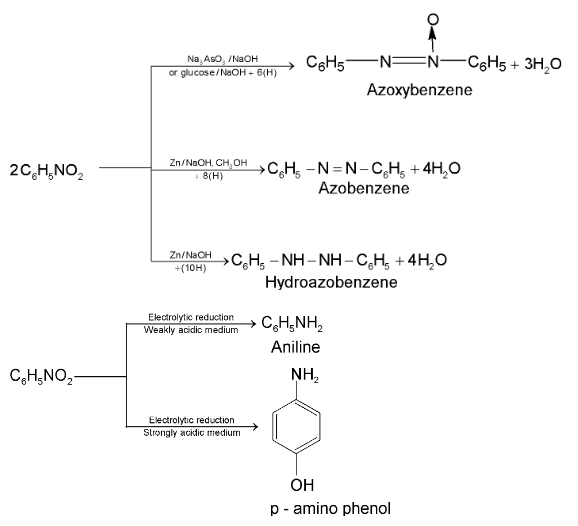
Very mild reducing agents like 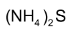 are used to reduce one nitro group without affecting the other.
are used to reduce one nitro group without affecting the other.
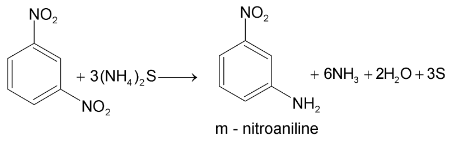
This reduction is called selective reduction named as Zinin reduction.
Illustration 2. Nitrobenzene when reduced with Zn and 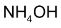 yields a product (A). Identify (A). Will (A) reduce Tollen’s reagent?
yields a product (A). Identify (A). Will (A) reduce Tollen’s reagent?
Solution: The product (A) is phenyl hydroxylamine. It reduces Tollen’s reagent.
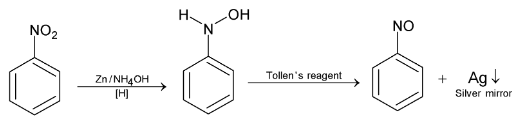
This reaction is Mulliken Test for distinguishing 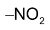 group.
group.
